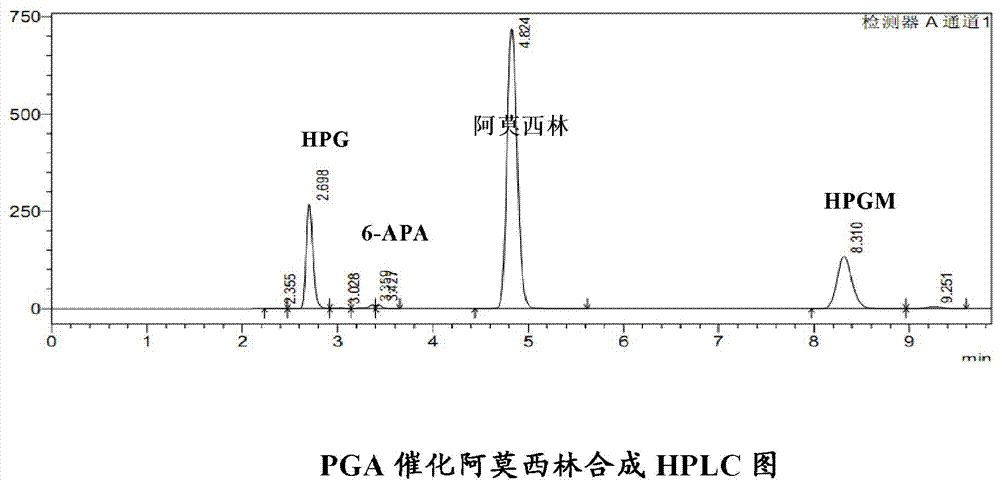
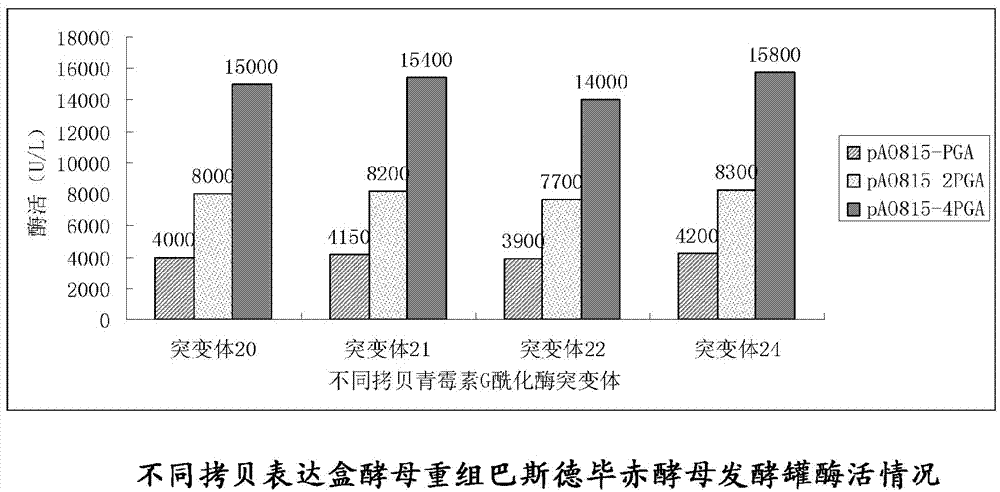
Penicillin G acylase containing one or a plurality of point mutation
A technology of penicillin and acylase, which is applied in the field of penicillin G acylase and its application in the production of β-lactam antibiotics, can solve the problems of reduced synthetic activity, many by-products, and low synthetic activity, and achieve the goal of producing The effect of increasing the amount of enzyme, reducing the cost and saving raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 Construction, prokaryotic expression and functional identification of the gene encoding the penicillin G acylase mutant
[0075] 1. Primer Design
[0076] will be on GeneBank E. coli The penicillin G acylase derived from ATCC11105 is a gene sequence, which is synthesized from the whole gene and primers are designed.
[0077] The primer sequence used for cloning in the prokaryotic expression vector pET-24a is:
[0078] Primer P1: 5'-ACCG CATATG GAGCAGCTTAGTTCAGAAATC-3' (the underlined base is the NdeI recognition site);
[0079] Primer P2: 5'-ACCG AAGCTT ATCTCTGGACGTGAAGGAC -3' (the underlined base is the HindIII recognition site); the penicillin G acylase gene sequence amplified by the pair of primers is shown in SEQ ID NO.2, and the penicillin G acylase protein encoded by it has Amino acid sequence shown in SEQ ID NO.1.
[0080] The primer sequences for the expression vector pAO815 clone in yeast are:
[0081] Primer P3: 5'-ACCG GAATTC GAGCAGCTTA...
Embodiment 2
[0139] Example 2 Eukaryotic expression and functional identification of penicillin G acylase mutant
[0140] Four copies of yeast expression vectors of mutants 20, 21, 22, and 24 were respectively constructed and transformed into Pichia pastoris cells. Mutant 20 was used as an illustration below, and the construction methods of other mutants were the same.
[0141] 1. Eukaryotic expression of penicillin G acylase mutants
[0142] The PGA gene of mutant 20 was introduced into the yeast expression vector pAO815 (invitrogen company) using the EcoRI restriction sites carried by primers P3 and P4, and transformed into E. coli In the TOP10, the E. coli TOP10 (pAO815-PGA) was cultured overnight in LB liquid medium at 37°C with shaking at 160 rpm, and the recombinant plasmid was extracted. The recombinant plasmid pAO815-PGA was linearized with SalI.
[0143] The linearized recombinant plasmid pAO815-PGA was transformed into yeast cells.
[0144] Preparation of Pichia pastoris GS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com