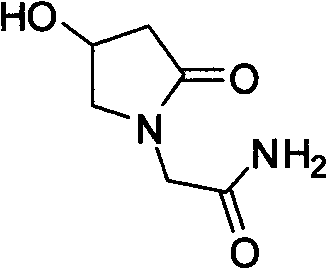
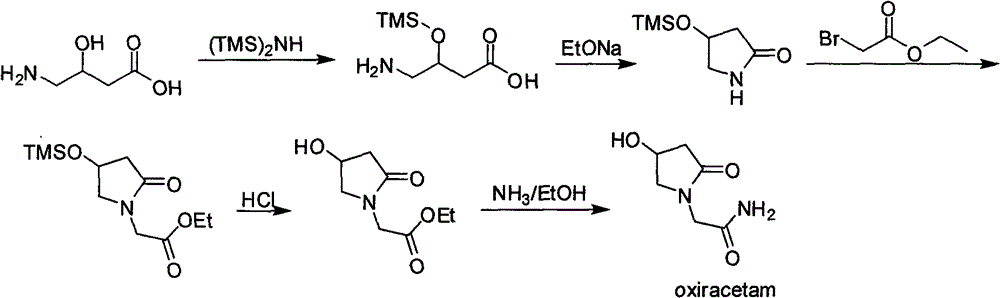
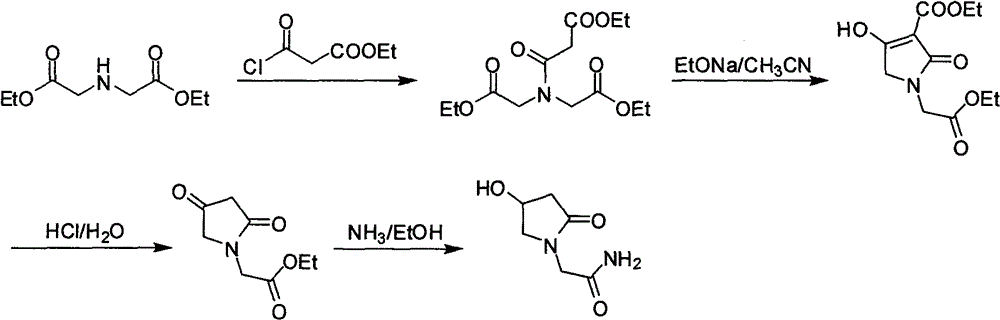
Oxiracetam preparation technology
A preparation process and intermediate technology, applied in the field of oxiracetam preparation process, can solve the problems of yield and product quality impact, salt is not easy to remove, etc., to achieve excellent product quality, avoid cumbersome post-processing, and less side reactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Get 171 grams (1mol) of 4-methoxyl-pyrroline-2-one-1-yl acetic acid (Guangan Kate Pharmaceutical Chemical Co., Ltd., the same below) and dissolve it in 1350ml of water, add 292 grams (2.8mol) of 35% hydrochloric acid , heated to 70°C and stirred for 3 hours to obtain an aqueous solution of Intermediate 1.
[0046] Cool the aqueous solution of Intermediate 1 to 0°C, slowly add 560 g of 20% sodium hydroxide solution, control the temperature at 0-5°C, and adjust the pH value to 3.0-4.0. Add 270 grams of 30% potassium borohydride solution dropwise, and control the temperature at about 5°C. After the dropwise addition, react at room temperature for 3 hours, then add 35% hydrochloric acid dropwise, adjust the pH value to 2.5-3.0, evaporate the solvent under reduced pressure, and obtain intermediate 2.
[0047] Intermediate 2 was dissolved in 1200 ml of methanol, 19.6 g (0.20 mol) of concentrated sulfuric acid was added, and refluxed for 8 hours to obtain an alcoholic solutio...
Embodiment 2
[0051] Dissolve 171 grams (1mol) of 4-methoxy-pyrrolin-2-one-1-ylacetic acid in 1300ml of water, add 189 grams (3mol) of nitric acid, heat up to 80°C, and stir for 2 hours to obtain the intermediate 1 in water.
[0052] Cool the aqueous solution of Intermediate 1 to 0°C, slowly add 600 g of 20% sodium hydroxide solution, control the temperature at 0-5°C, and adjust the pH value to 3.0-4.0. Add 288 grams of 30% potassium borohydride solution dropwise, and control the temperature at about 5°C. After the dropwise addition, react at room temperature for 3 hours, add nitric acid, adjust the pH value to 2.5-3.0, evaporate the solvent under reduced pressure, and obtain intermediate 2.
[0053]Intermediate 2 was dissolved in 1180 ml of methanol, 18.5 g (0.19 mol) of concentrated sulfuric acid was added, and refluxed for 8 hours to obtain an alcoholic solution of intermediate 3.
[0054] Pass the alcohol solution of intermediate 3 into 68 g (4 mol) of ammonia gas, react at room tempe...
Embodiment 3
[0056] Dissolve 171 grams (1mol) of 4-methoxy-pyrroline-2-one-1-ylacetic acid in 1200ml of water, add 245 grams (2.5mol) of concentrated sulfuric acid, heat up to 75°C, and stir for 3 hours. An aqueous solution of Intermediate 1 was obtained.
[0057] Cool the aqueous solution of Intermediate 1 to 0°C, slowly add 500 g of 20% sodium hydroxide solution, control the temperature at 0-5°C, and adjust the pH value to 3.0-4.0. 270 grams of 30% sodium borohydride solution was added dropwise, and the temperature was controlled at about 5°C. After the dropwise addition, react at room temperature for 2.5 hours, add sulfuric acid, adjust the pH value to 2.5-3.0, evaporate the solvent under reduced pressure, and obtain intermediate 2.
[0058] Intermediate 2 was dissolved in 1120 ml of methanol, 19.6 g (0.2 mol) of concentrated sulfuric acid was added, and refluxed for 8 hours to obtain a methanol solution of intermediate 3.
[0059] Pass the methanol solution of intermediate 3 into 63 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com