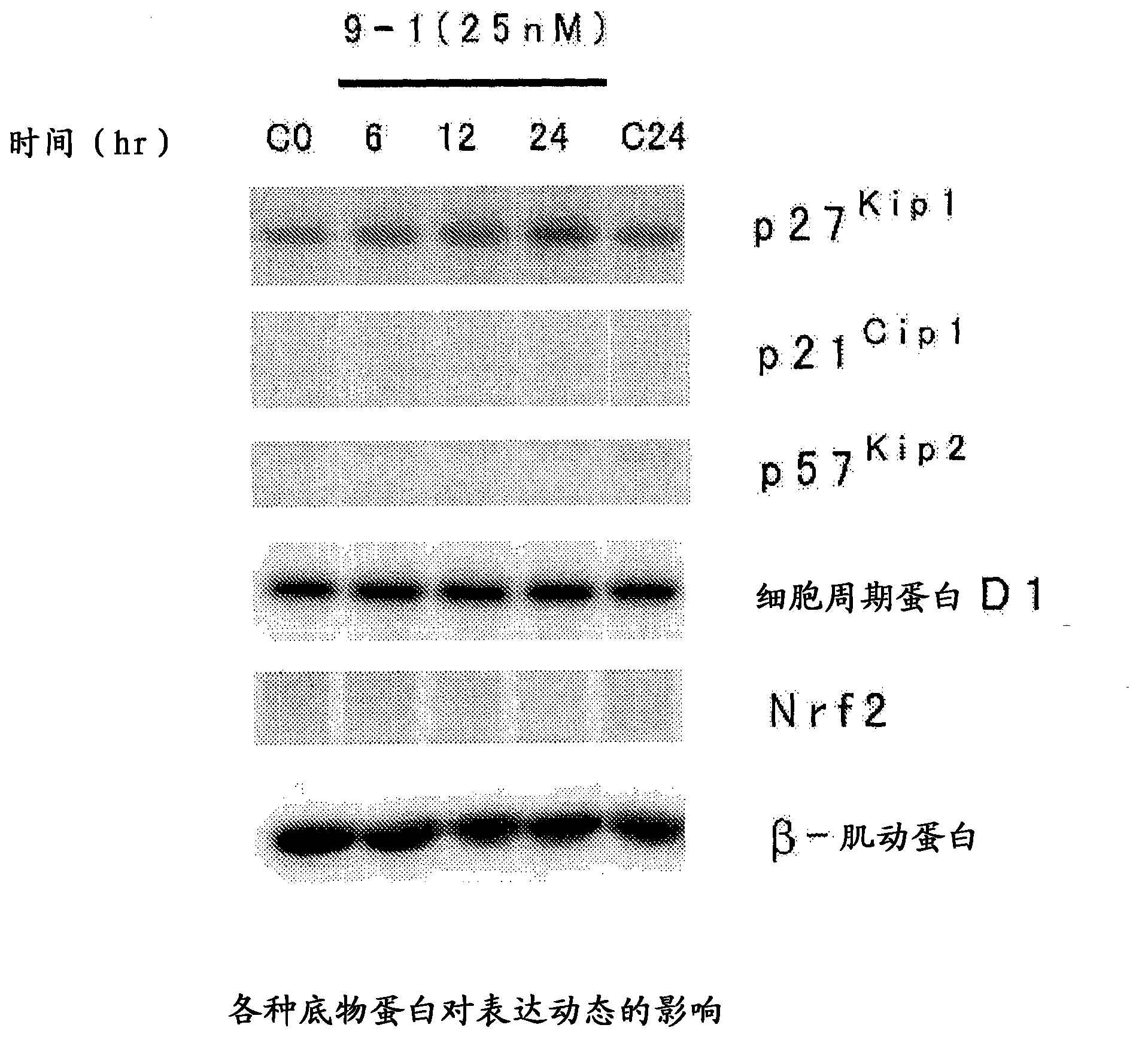
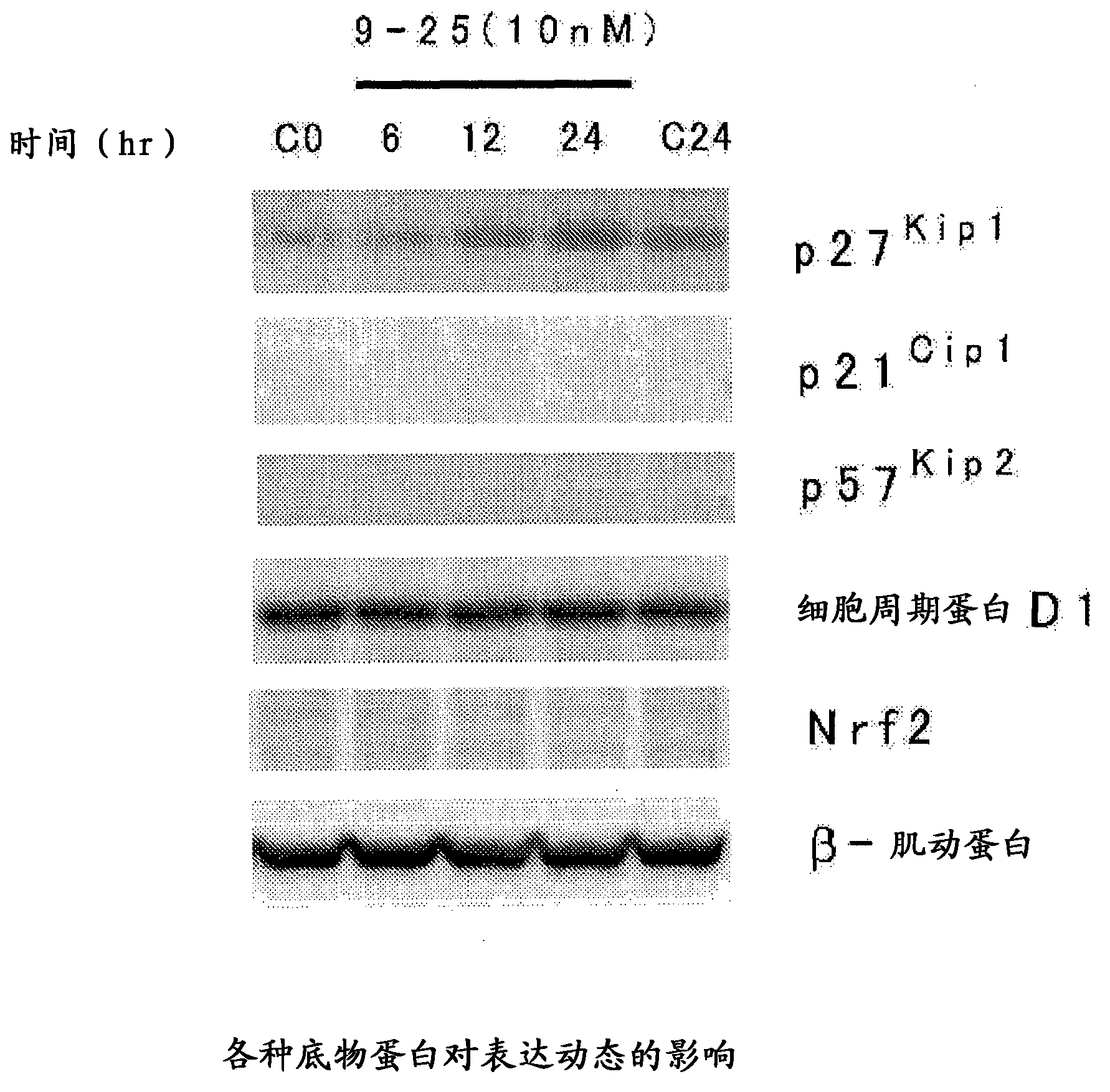
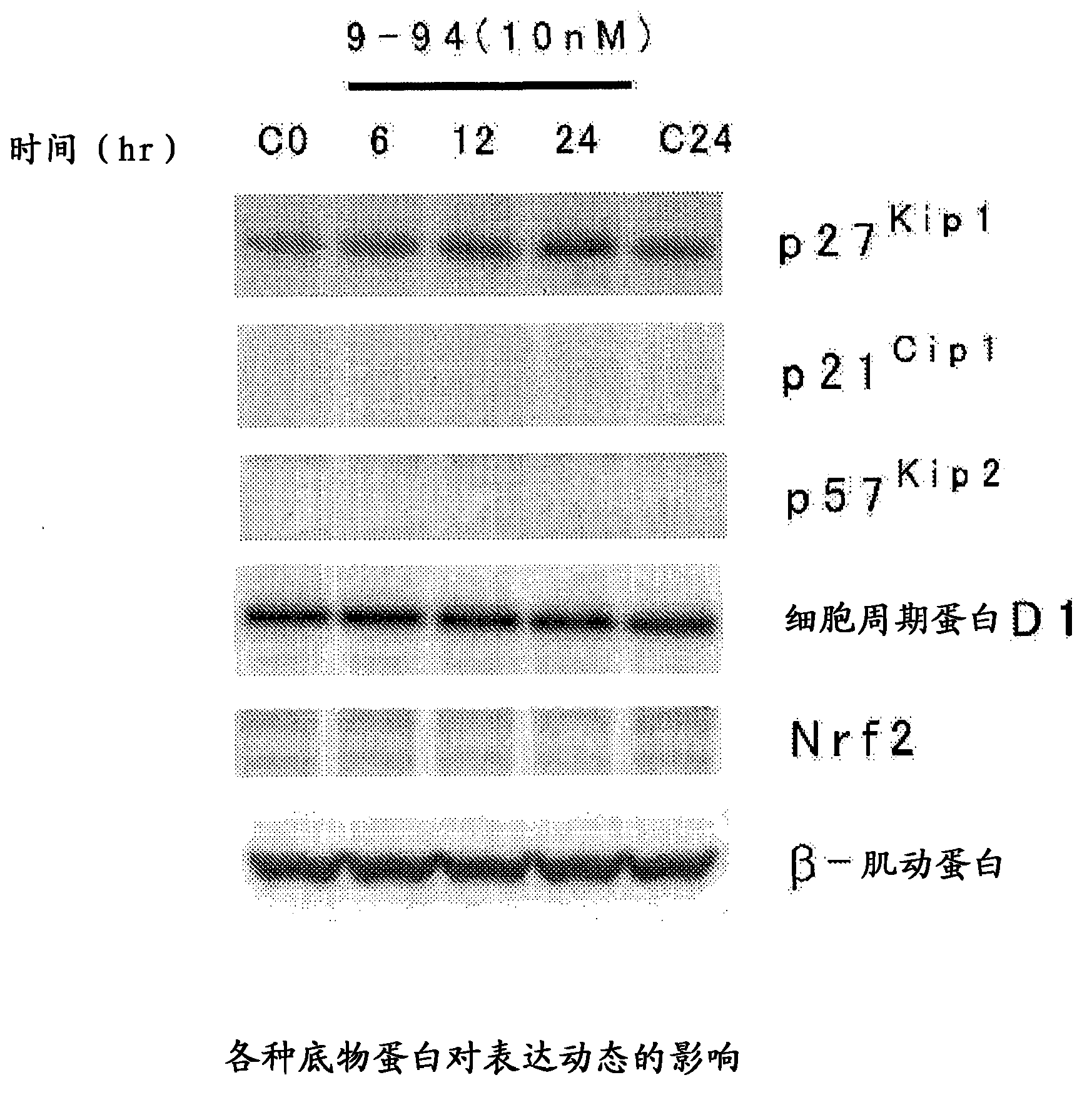
Heterocyclic compound, and p27 kip1 degradation inhibitor
A compound and heterocyclic technology, applied in the field of heterocyclic compounds and p27Kip1 decomposition inhibitors, can solve problems such as undocumented relationships
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0324] Step 1-1-1
[0325] Ethyl 4-fluoro-3-nitrobenzoate
[0326] [chem 30]
[0327]
[0328] Suspend 4-fluoro-3-nitrobenzoic acid (150g, 0.810mol) in ethanol (1000ml), add concentrated sulfuric acid (25ml) dropwise, and heat to reflux for 8 hours. After standing to cool, it was concentrated under reduced pressure, and water was added with stirring. The precipitate was separated by filtration, washed with water and ventilated to dry to obtain the title compound (160 g, 93%) as a yellow solid.
[0329] 1 H-NMR (DMSO-d 6 ) δ: 8.56 (dd, J=2.3, 7.3Hz, 1H), 8.35-8.31 (m, 1H), 7.76-7.71 (m, 1H), 4.37 (q, J=7.3Hz, 2H), 1.35 (t , J=7.3Hz, 3H).
[0330] Mass spectrum, m / z: 213 (M + ), 185, 168 (base peak).
[0331] Step 1-1-2
[0332] 4-Methylamino-3-nitrobenzoic acid ethyl ester
[0333] [chem 31]
[0334]
[0335] Dissolve ethyl 4-fluoro-3-nitrobenzoate (10.0 g, 46.9 mmol) prepared in the above step 1-1-1 in methanol (40 ml), add triethylamine (10 ml, 70.4 mmol), an...
Embodiment 1-2~1-6
[0360] Instead of the 40% methylamine-methanol solution as the ring-forming component, or ethyl 4-fluoro-3-nitrobenzoate as the monocyclic compound, the compounds shown in the table below were used. 1-1 is operated in the same way to obtain the target bicyclic compound.
[0361] [Table 9]
[0362] Table 9
[0363]
[0364] Synthetic route 2
[0365] [chem 35]
[0366]
[0367] (In the formula, R represents an alkyl group, and R' represents an alkyl or alkoxyalkyl group)
Embodiment 2-1
[0369] Step 2-1-1
[0370] 1,2-Dimethyl-1H-benzimidazole-5-carboxylic acid ethyl ester
[0371] [chem 36]
[0372]
[0373] Ethyl 3-amino-4-methylaminobenzoate (1.00 g, 5.15 mmol) prepared in step 1-1-3 was dissolved in acetic anhydride (4 ml), and heated under reflux for 19 hours. After standing to cool, it was neutralized with saturated sodium bicarbonate solution, and extracted with ethyl acetate. Dry over anhydrous magnesium sulfate, then concentrate. Purification by silica gel column chromatography (chloroform:methanol=10:1) gave the title compound (1.15 g, quantitative) as a light brown oil.
[0374] 1 H-NMR (CDCl 3 ) δ: 8.39 (d, J=1.5Hz, 1H), 7.98 (dd, J=1.5, 8.5Hz, 1H), 7.28 (d, J=8.5Hz, 1H), 4.39 (q, J=6.9Hz, 2H), 3.75 (s, 3H), 2.62 (s, 3H), 1.41 (t, J=6.9Hz, 3H).
[0375] Mass spectrum, m / z: 218 (M + ), 173 (base peak).
[0376] Step 2-1-2
[0377] (1,2-Dimethyl-1H-benzimidazol-5-yl)methanol
[0378] [chem 37]
[0379]
[0380] Using ethyl 1,2-dimet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com