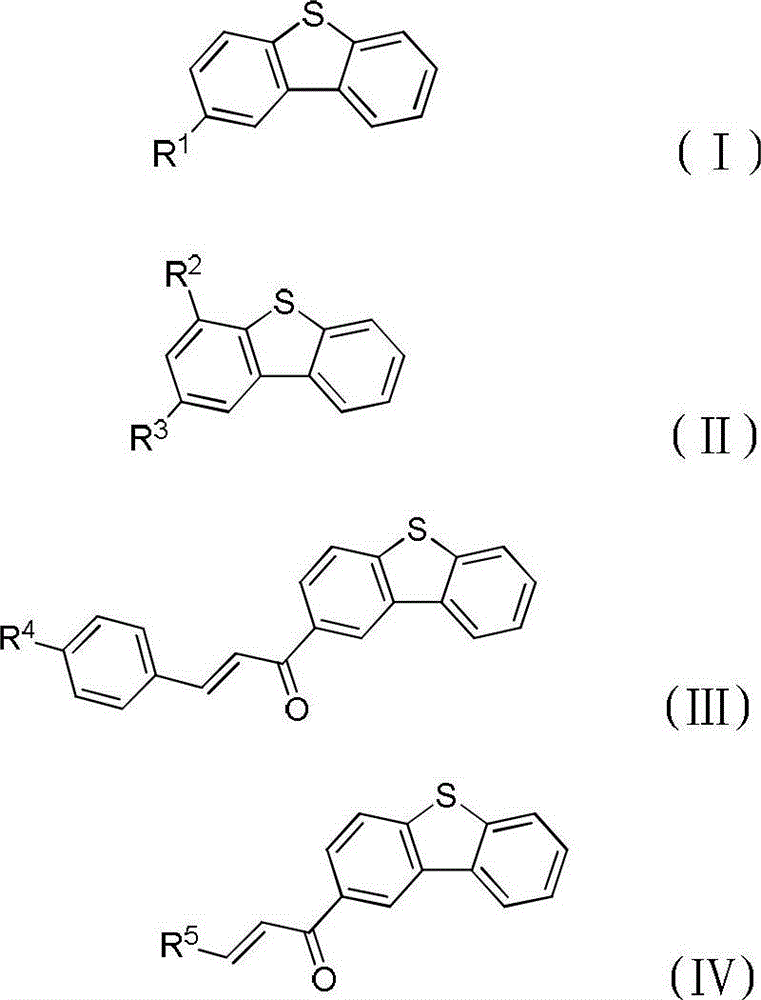
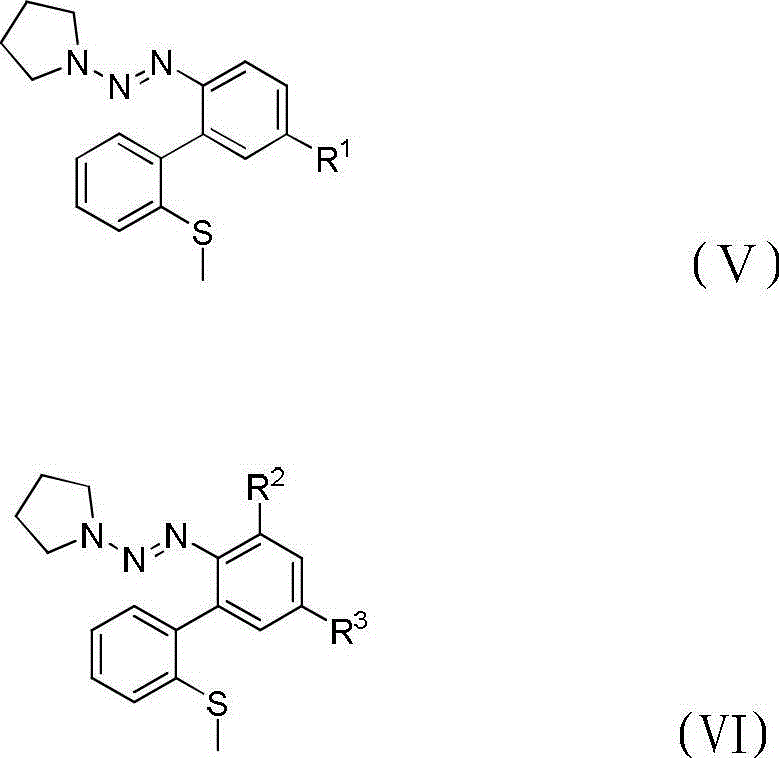
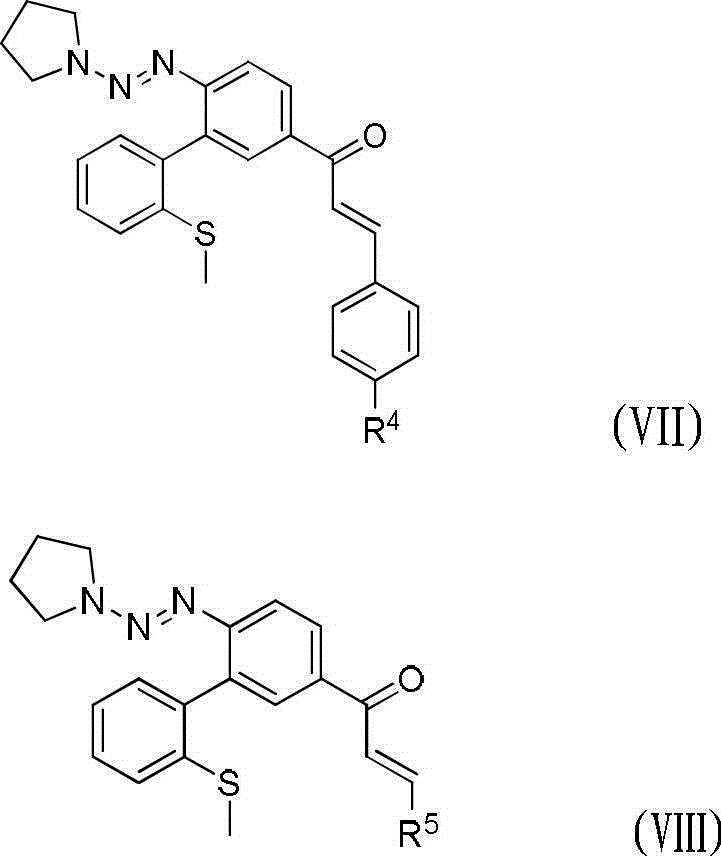
Method for preparing dibenzothiophene derivatives
A technology for dibenzothiophene and derivatives is applied in the field of preparation of dibenzothiophene derivatives and achieves the effects of low reaction cost, simple process and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 100 mL reaction flask, add 369 mg of methylthio-containing triazene 1a and 40 mL of acetonitrile, and then slowly add 0.256 mL of boron trifluoride diethyl ether dropwise with a 1 mL syringe. The reaction solution was stirred at 45°C for 4 hours. After the reaction, cool to room temperature, filter with a glass funnel, and purify the filtrate through a silica gel column (the volume ratio of petroleum ether and ethyl acetate is 10:1) to obtain 215 mg of the product with a yield of 80%. The reaction process is shown in the following formula :
[0029]
[0030] The product prepared in the present embodiment is carried out nuclear magnetic resonance analysis, and the results are as follows:
[0031] 1 H NMR (400 MHz, CDCl 3 ): δ 8.84(s, 1H), 8.22-8.26(m, 1H), 8.11-8.14(m, 1H), 7.84-7.90(m, 2H), 7.48-7.51(m, 2H), 4.46(q , J=6.8Hz, 2H), 1.47(t, J=7.2Hz, 3H);
[0032] 13 C NMR (100 MHz, CDCl 3 ): δ 166.7, 144.2, 139.6, 135.5, 135.2, 127.3, 126.8, 124.8, 123.1, 1...
Embodiment 2
[0034] In a 100 mL reaction flask, add 369 mg of methylthio-containing triazene 1a and 40 mL of methanol, and then slowly add 0.256 mL of boron trifluoride diethyl ether dropwise with a 1 mL syringe. The reaction solution was stirred at 45°C for 4 hours. After the reaction, cool to room temperature, filter with a glass funnel, and purify the filtrate through a silica gel column (the volume ratio of petroleum ether and ethyl acetate is 10:1) to obtain 166 mg of the product with a yield of 65%. The reaction process is shown in the following formula :
[0035]
[0036] The product prepared in the present embodiment is carried out nuclear magnetic resonance analysis, and the results are as follows:
[0037] 1 H NMR (400 MHz, CDCl 3 ): δ 8.84(s, 1H), 8.22-8.26(m, 1H), 8.11-8.14(m, 1H), 7.84-7.90(m, 2H), 7.48-7.51(m, 2H), 4.46(q, J=6.8Hz, 2H), 1.47(t, J=7.2Hz, 3H);
[0038] 13 C NMR (100 MHz, CDCl 3 ): δ 166.7, 144.2, 139.6, 135.5, 135.2, 127.3, 126.8, 124.8, 123.1, 122.8,...
Embodiment 3
[0040] In a 100 mL reaction flask, add 369 mg of methylthio-containing triazene 1a and 40 mL of toluene, and then slowly add 0.256 mL of boron trifluoride diethyl ether dropwise with a 1 mL syringe. The reaction solution was stirred at 45°C for 4 hours. After the reaction, cool to room temperature, filter with a glass funnel, and purify the filtrate through a silica gel column (the volume ratio of petroleum ether and ethyl acetate is 10:1) to obtain 103 mg of the product with a yield of 40%. The reaction process is shown in the following formula :
[0041]
[0042] The product that present embodiment prepares is carried out nuclear magnetic resonance analysis, and the result is as follows:
[0043] 1 H NMR (400 MHz, CDCl3 ): δ 8.84(s, 1H), 8.22-8.26(m, 1H), 8.11-8.14(m, 1H), 7.84-7.90(m, 2H), 7.48-7.51(m, 2H), 4.46(q, J=6.8Hz, 2H), 1.47(t, J=7.2Hz, 3H);
[0044] 13 C NMR (100 MHz, CDCl 3 ): δ 166.7, 144.2, 139.6, 135.5, 135.2, 127.3, 126.8, 124.8, 123.1, 122.8, 122.5,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com