Synthesis method of N-methyl-2-fluoroaniline
A synthesis method and technology of fluoroaniline, applied in the field of synthesis of N-methyl-2-fluoroaniline, can solve the problems of unfavorable industrial production, unfriendly environment, high cost, etc., achieve high selectivity, cheap and easy-to-obtain reaction raw materials, good quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
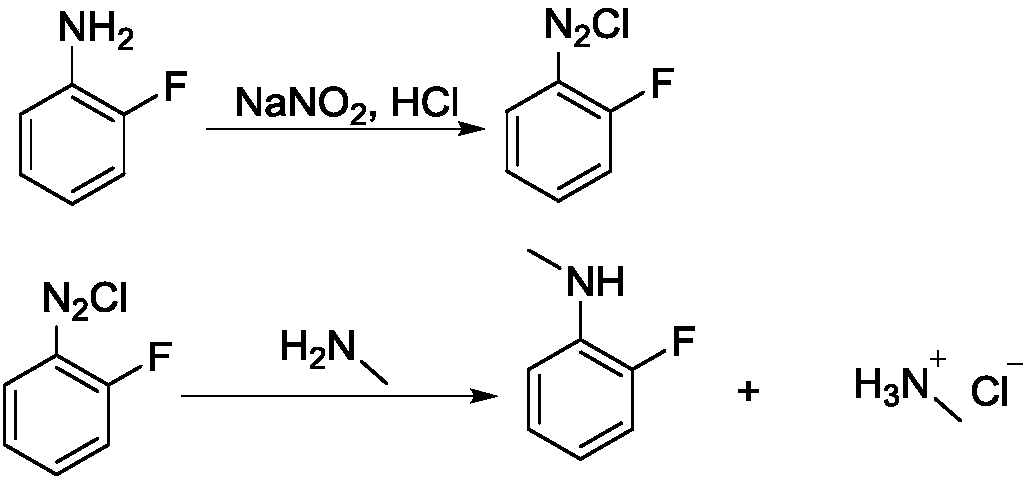
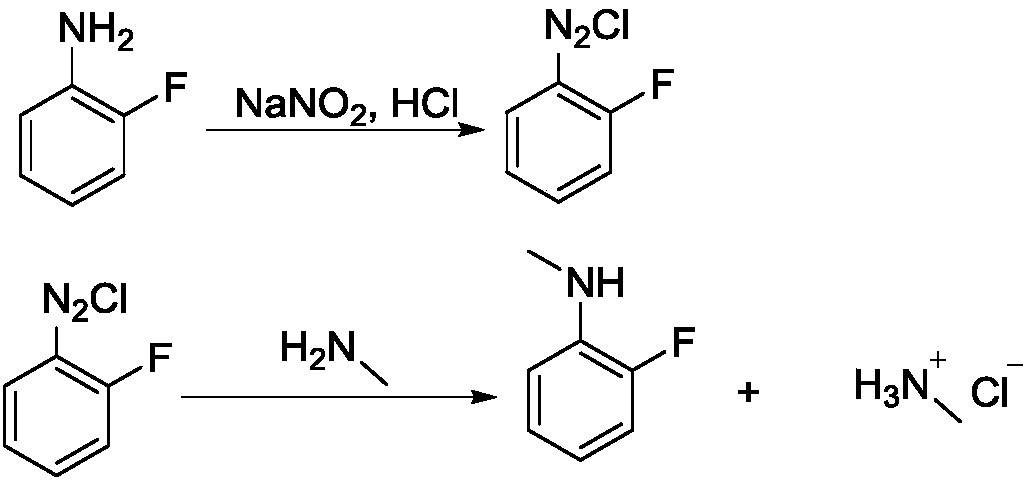
[0024] Step 1, diazotization reaction
[0025] Add 27.8g of 2-fluoroaniline and 90g of 30% hydrochloric acid into a 250mL four-necked bottle, stir for 30min, place the system in an ice-salt bath to cool down to the internal temperature of -10°C, and dropwise add sodium nitrite solution (19g of sodium nitrite Dissolve in 50mL water), keep the internal temperature below 5°C, and drop it in about 1 hour; after the dropwise addition, keep it warm for 30 minutes and blow out the residual HCl with nitrogen, and the system is ready for use.
[0026] Step 2, methylation reaction
[0027] Heat 60 g of methylamine 40% aqueous solution to 50° C., add dropwise the above-prepared 2-fluoroaniline diazonium salt solution into the reaction flask, a large amount of gas is released, and the dropwise addition is completed in about 2 hours. After the dropwise addition, stir until the reaction is complete, about 30 minutes. After the reaction was completed, the organic solvent ethyl acetate (EA)...
Embodiment 2
[0029] Prepare the synthetic method of N-methyl-2-fluoroaniline with the method of embodiment 1, difference is:
[0030] Group A: In the step 1, before adding the sodium nitrite solution, the system was cooled to -15°C; the yield of N-methyl-2-fluoroaniline was 95%;
[0031] Group B: in the step 1, before adding the sodium nitrite solution, the system was cooled to -5°C; the yield of N-methyl-2-fluoroaniline was 88%;
[0032] Group C: in the step 1, before adding the sodium nitrite solution, the system was cooled to 0°C; the yield of N-methyl-2-fluoroaniline was 78%;
Embodiment 3
[0034] Prepare the synthetic method of N-methyl-2-fluoroaniline with the method of embodiment 1, difference is:
[0035] Group A: In the step 1, the temperature of the heat preservation reaction is: -10°C, and the reaction time is 2 hours; the yield of N-methyl-2-fluoroaniline is 94%;
[0036] Group B: In the step 1, the temperature of the heat preservation reaction is: 15° C., and the reaction time is 2 hours; the yield of N-methyl-2-fluoroaniline is 45% (GC yield);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com