Preparation method of spherical ferrite
A technology of ferrite and ferric chloride, which is applied in the preparation of manganese ferrite and magnesium ferrite powder, and in the field of spherical spinel zinc ferrite, can solve the problems of difficult control of material morphology, Achieve the effect of easy control of reaction conditions, simple reaction raw materials and good crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
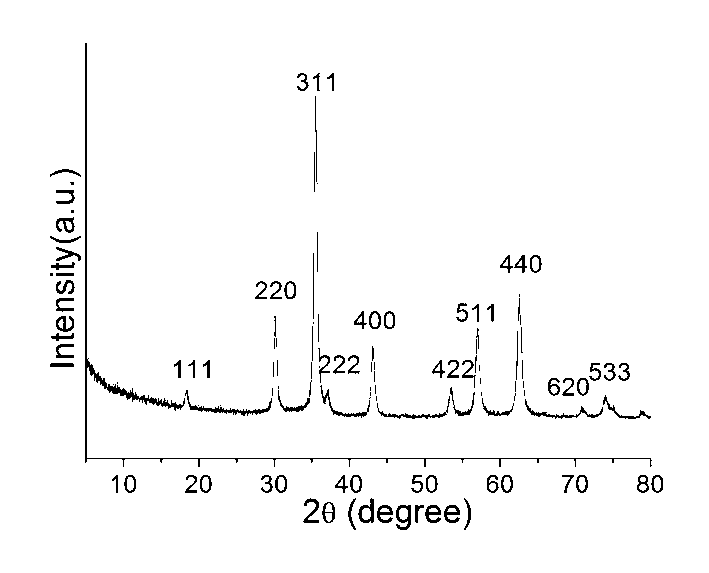
[0025] At room temperature, 1.0812g FeCl 3 ·6H 2 O and 0.2726g ZnCl 2 Dissolve in 50mL ethylene glycol solution, stir and dissolve at room temperature to obtain a uniformly dispersed solution. Measure 2mL of ethanolamine into the solution, stir and mix evenly, transfer the solution into a reaction kettle (the capacity of the reaction kettle is 100mL), and seal it. The reaction kettle was put into an oven and kept at 200° C. for 24 hours. After the reaction kettle was naturally cooled to room temperature, the product was taken out, and the product was separated by centrifugation. The separated product was washed three times with deionized water and absolute ethanol respectively, and dried in the air at 60°C. figure 1 The result of X-ray powder diffraction shows that it is a single-phase zinc ferrite. from figure 2 It can be seen from the transmission electron microscope photos that the zinc ferrite is spherical in shape with a particle size of 100-300nm.
Embodiment 2
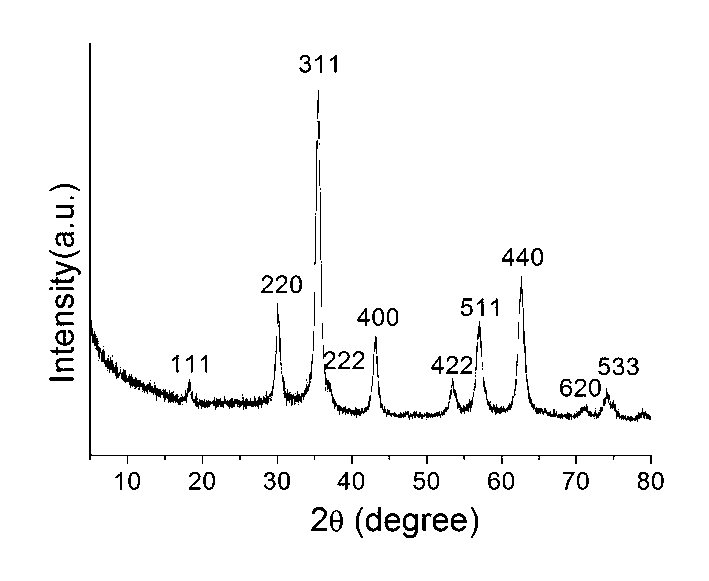
[0027] At room temperature, 1.0812g FeCl 3 ·6H 2 O and 0.2726g ZnCl 2 Dissolve in 50mL ethylene glycol solution, stir and dissolve at room temperature to obtain a uniformly dispersed solution. Measure 5mL of ethanolamine and add it into the solution, stir and mix evenly, transfer the solution into a reaction kettle (the capacity of the reaction kettle is 100mL), and seal it. Reactor is put into baking oven, is incubated at 200 ℃ for 12 hours. After the reaction kettle was naturally cooled to room temperature, the product was taken out, and the product was separated by centrifugation. The separated product was washed three times with deionized water and absolute ethanol respectively, and dried in the air at 60°C. image 3 The result of X-ray powder diffraction shows that it is a single-phase zinc ferrite.
Embodiment 3
[0029] At room temperature, 1.0812g FeCl 3 ·6H 2 O and 0.2726g ZnCl 2 Dissolve in 50mL ethylene glycol solution, stir and dissolve at room temperature to obtain a uniformly dispersed solution. Measure 10mL of ethanolamine into the solution, stir and mix evenly, transfer the solution into a reaction kettle (the capacity of the reaction kettle is 100mL), and seal it. The reaction kettle was put into an oven and kept at 180° C. for 24 hours. After the reaction kettle was naturally cooled to room temperature, the product was taken out, and the product was separated by strong magnet adsorption. The separated product was washed 3 times with deionized water and absolute ethanol respectively, and dried in the air at 60°C. Figure 4 The result of X-ray powder diffraction shows that it is a single-phase zinc ferrite.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com