Composite polyepoxy chloropropane alkaline polymer membrane electrode and preparation method thereof
A polyepichlorohydrin, polymer film technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of AAEM's poor high temperature resistance, poor chemical stability, low mechanical strength, etc., to avoid limited contact interface, The preparation method is simple and the effect of improving the conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Polyepichlorohydrin (PECH) was dissolved in N,N-dimethyl sulfoxide (DMSO), heated with stirring, to a temperature of 100 °C; under an inert atmosphere, 30% N, N,N`,N`-tetramethyl-1,6-ethylenediamine was reacted for 48 hours. After the reaction was completed, the solution was removed, filtered and used for later use to obtain quaternized polyepichlorohydrin (QPECH); to the above homogeneous solution Add a certain amount of ethanol and acetone, stir for 2 hours to form a 14% uniform film-forming solution; cast the film-forming solution into the pretreated porous PTFE film, heat at 60 °C to evaporate the solvent, and at 100 °C After 12 hours of vacuum heat treatment, the desired anion exchange membrane was obtained.
[0041] The carbon paper was treated with polytetrafluoroethylene (PTFE) emulsion and sintered, firstly sintered at 120 °C for 15 min, and then at 350 °C for 15 min; then a certain amount of carbon powder and polyperfluoroethylene propylene (FEP) emulsion were...
Embodiment 2
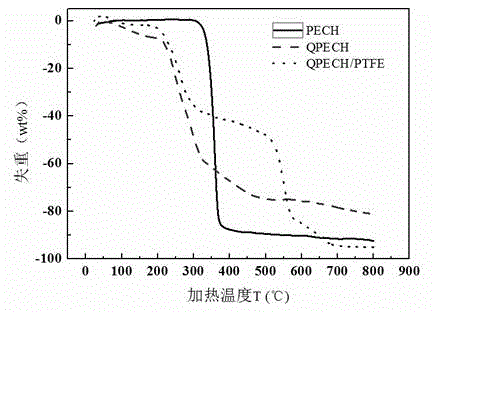
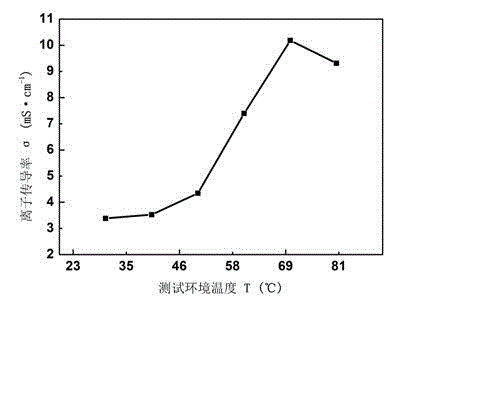
[0046] The preparation process of the anion exchange membrane is as described in Example 1, except that the tertiary amine is N,N,N',N'-tetramethyl-1,6-hexanediamine. Thermogravimetric analysis tests were performed on polyepichlorohydrin (PECH), synthetic quaternized polyepichlorohydrin (QPECH), prepared anion exchange membranes (QPECH / PTFE), such as figure 1 As shown in the figure, the prepared anion exchange membrane (QPECH / PTFE) has a decomposition temperature greater than 200 °C, has good thermal stability, and can meet the needs of low-temperature fuel cell operation (the working temperature is generally lower than 120 °C); The ionic conductivity of the exchange membrane was tested by testing such as figure 2 As shown, the prepared anion exchange membrane has high ionic conductivity, and the highest ionic conductivity is greater than 10 -2 S cm -1 (Currently the advanced level of anion exchange membranes is 10 -2 magnitude).
[0047] The preparation process of the g...
Embodiment 3
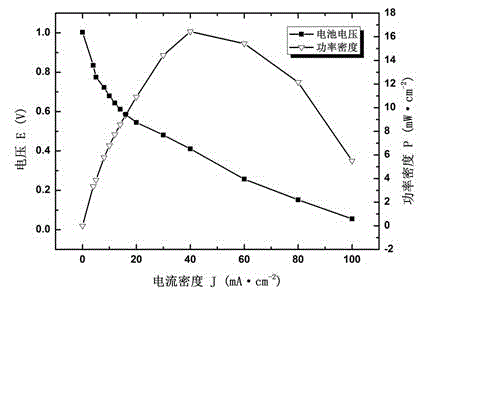
[0051] The membrane electrode in Example 2 was applied to the fuel cell, and the polarization characteristic curve test was carried out. The intake air metering ratio is 2.0. Test results such as image 3 It shows that the prepared composite polyepichlorohydrin alkaline polymer membrane electrode exhibits good electrical performance of alkaline fuel cells, the battery open circuit voltage is greater than 1.0V, and the maximum power density is 16.4 mW cm -2 . This example is the application of the prepared membrane electrode in a fuel cell. The electrochemical performance of the fuel cell is expressed by the polarization curve. The results show that the prepared membrane electrode can operate normally in the fuel cell; Compared with the performance of membrane fuel cells, the performance is relatively good.
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| ionic conductivity | aaaaa | aaaaa |
| open-circuit voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com