Method for separating chiral compound by using molecular sieves
A chiral compound and separation method technology, applied in the field of pharmaceutical synthesis and organic compound synthesis and preparation, can solve the problems of difficult high-purity products, large amount of solvents, low production volume, etc., achieve rapid and effective separation, shorten production cycle, and reduce production pollution small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
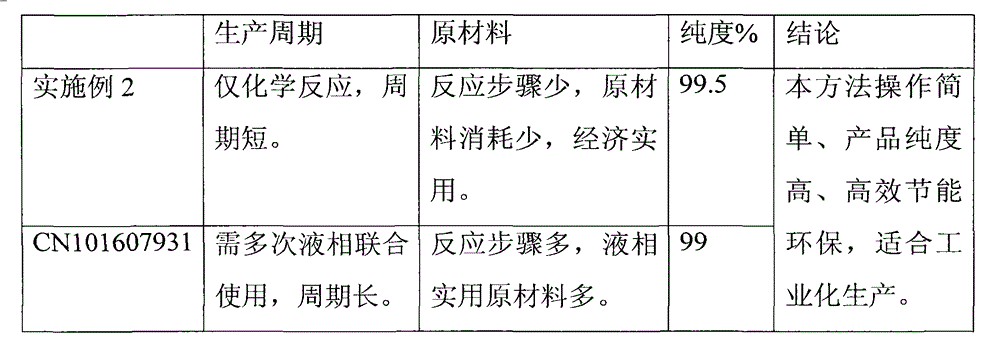
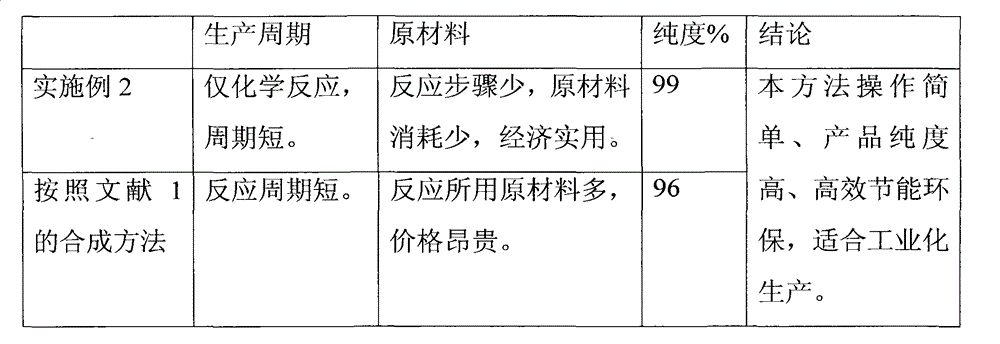
Examples
Embodiment 1
[0018] Get molecular sieve zeolite (molecular formula: 4K 2 O·2Na 2 O·6Al 2 o 3 12SiO2 2 27H 2 O, the effective pore diameter is about 0.3nm) placed in glacial acetic acid, take 1α, 25-(OH) 2 -cycling-VD 3 The sample was stirred and dissolved in glacial acetic acid at 30°C, continued to stir for 15 minutes, sealed and stood for 1 hour, extracted three times with ethyl acetate, combined and concentrated the extracts, dissolved in absolute ethanol, and dissolved sodium hydroxide in Add methanol dropwise, stir at room temperature for 1 hour, extract three times with ethyl acetate after the reaction is complete, combine the extracts and wash with saturated aqueous sodium chloride until neutral, dry, filter, evaporate the filtrate to dryness and dissolve in ethyl acetate Add n-pentane to the ester, cool to room temperature and let stand for 12 hours. Acicular crystals are precipitated. The crystals are collected by filtration and vacuum-dried to obtain calcitriol with a purit...
Embodiment 2
[0020] Get molecular sieve zeolite (molecular formula: 2Na 2 O·2Al 2 o 3 4SiO2 2 9H 2 O, the effective pore diameter is about 0.4nm) placed in glacial acetic acid, take 1α, 25-(OH) 2 -cycling-VD 3 The sample was stirred and dissolved in glacial acetic acid at 30°C, continued to stir for 15 minutes, sealed and stood for 1 hour, extracted three times with ethyl acetate, combined and concentrated the extracts, dissolved in absolute ethanol, and dissolved sodium hydroxide in Add methanol dropwise, stir at room temperature for 1 hour, extract three times with ethyl acetate after the reaction is complete, combine the extracts and wash with saturated aqueous sodium chloride until neutral, dry, filter, evaporate the filtrate to dryness and dissolve in ethyl acetate Add n-pentane to the ester, cool to room temperature and let it stand for 12 hours. Acicular crystals precipitate out. The crystals are collected by filtration and vacuum-dried to obtain calcitriol with a purity of 99....
Embodiment 3
[0026] Get molecular sieve zeolite (molecular formula: 4K 2 O·2Na 2 O·6Al 2 o 3 12SiO2 2 27H 2 O, the effective pore diameter is about 0.3nm) placed in glacial acetic acid, take 1α-OH-3,5-cyclo-VD 3 The sample was stirred and dissolved in glacial acetic acid under the condition of 55°C, continued to stir for 25 minutes, sealed and stood for 1 hour, the reaction solution was neutralized with saturated aqueous sodium bicarbonate solution, the pH value was adjusted to 8.0, extracted three times with ether, and the ether layer was washed with water To neutrality, dehydrate with anhydrous magnesium sulfate, filter and concentrate, dissolve in absolute ethanol, and dissolve sodium hydroxide in methanol, add it dropwise, stir at room temperature for 30 minutes, add ethyl acetate dropwise to extract after the reaction is complete Three times, the extracts were combined and washed with saturated aqueous sodium chloride until neutral, dehydrated with anhydrous magnesium sulfate and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com