Gemini surface active agent using pyrrole ring as hydrophilic head group and preparation method of Gemini surface active agent using pyrrole ring as hydrophilic head group
A surfactant and pyrrole ring technology, applied in the field of surfactant preparation, can solve the problems of complex preparation steps of Gemini surfactant, unsuitable for large-scale production and application, surface activity needs to be improved, etc., and achieve low critical micelle concentration , strong industrial operability, good viscoelastic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
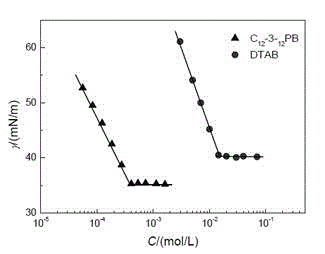
[0030] The preparation (C 8 -3- 8 PB)
[0031] Add 14.2g (0.2 moles) of pyrrolidine to 200ml of methanol, add 24g (0.6 moles) of sodium hydroxide and 84.5g (0.44 moles) of bromooctane in turn, and keep the reaction at 40°C for 6 hours; after the reaction is completed, depressurize Remove methanol and unreacted pyrrolidine, and the obtained light yellow liquid is separated by a chromatographic column filled with 40 times its weight of silica gel, and petroleum ether, dichloromethane, ethyl acetate / dichloromethane / ammonia water are used as eluents in sequence , collect the eluate and concentrate to obtain N-octylpyrrolidine with a yield of 96%. Add 250ml of isopropanol or acetonitrile and 13.3g (0.0665 moles) of 1,3-dibromopropane to the N-octylpyrrolidine obtained above, stir vigorously, and react at 100°C for 12 hours; Isopropanol, the obtained pale yellow solid was recrystallized with a mixed solvent of ethanol / acetone, and the resulting product was vacuum-dried to obtain ...
Embodiment 2
[0040] The preparation (C 12 -3- 12 PB)
[0041]Add 14.2g (0.2 moles) of pyrrolidine to 200ml of ethanol, add 32g (0.8 moles) of sodium hydroxide and 124g (0.5 moles) of dodecane bromide in turn, and keep it at 60°C for 10 hours; after the reaction is completed, depressurize Remove ethanol and unreacted pyrrolidine, and the obtained pale yellow liquid is separated by a chromatographic column filled with 40 times its weight of silica gel, and sequentially use n-hexane, dichloromethane, ethyl acetate / dichloromethane / ammonia water as eluent , collect the eluate and concentrate to obtain N-dodecylpyrrolidine with a yield of 98%. Add 250ml of isopropanol and 15.7g (0.0784 moles) of 1,3-dibromopropane to the N-dodecylpyrrolidine obtained above, stir vigorously, and react at 80°C for 48 hours; after the reaction, remove isopropanol under reduced pressure. propanol, the obtained light yellow solid is recrystallized with the mixed solvent of ethanol / ethyl acetate, and the product ob...
Embodiment 3
[0049] The preparation (C 18 -3- 18 PB)
[0050] Add 14.2g (0.2 mole) of pyrrole ring to 200ml of N,N-dimethylformamide, add 50.4g (0.6 mole) of sodium bicarbonate and 353g (0.8 mole) of octadecane bromide in sequence, and keep the reaction at 80°C 24 hours; after the reaction was completed, N,N-dimethylformamide and unreacted pyrrolidine were removed under reduced pressure, and the obtained light yellow liquid was separated by a chromatographic column equipped with 40 times its weight of silica gel, and then washed with n-hexane, Dichloromethane, ethyl acetate / dichloromethane / ammonia water were used as eluents, and the eluent was collected and concentrated to obtain N-octadecylpyrrolidine with a yield of 95%. Add 250ml of N,N-dimethylformamide and 13.3g (0.0665 moles) of 1,3-dibromopropane to the N-octadecylpyrrolidine obtained above, stir vigorously, and react at 120°C for 72 hours; After the end, N,N-dimethylformamide was removed under reduced pressure, and the wine-red ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com