10-hydroxycamptothecine nanometer microsphere and preparation method thereof
A technology of hydroxycamptothecin and nano-microspheres, which is applied in the field of biomedical materials, can solve the problems of instability, reduced curative effect, and limited dosing amount, achieves automatic and large-scale production, improves the scope of application, and prolongs the action time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
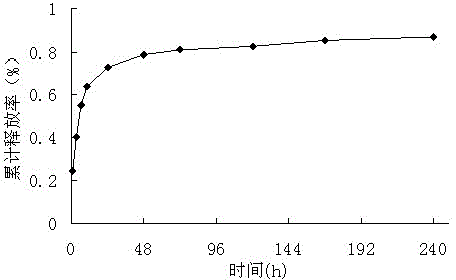
Examples
Embodiment 1
[0047] Precisely weigh 10mg10-hydroxycamptothecin bulk drug and 100mgPLGA.
[0048] Dissolve 10-hydroxycamptothecin and PLGA in 5 ml of methanol:dichloromethane (v / v = 1:3) binary solvent. After fully dissolving, the above organic solution was added dropwise to 10ml of a 2% polyvinyl alcohol aqueous solution, and ultrasonically emulsified for 30s under the condition of an amplitude of 38%. Subsequently, the emulsion obtained by emulsification was added to 85 ml of polyvinyl alcohol aqueous solution with a mass concentration of 0.2% to disperse and solidify. Stir at room temperature for 6h to completely evaporate the organic solvent. The cured microspheres were centrifuged at 4° C. at a rotational speed of 10,000 r / min for 10 minutes, collected, and freeze-dried by adding a lyoprotectant to obtain 10-hydroxycamptothecin microsphere powder.
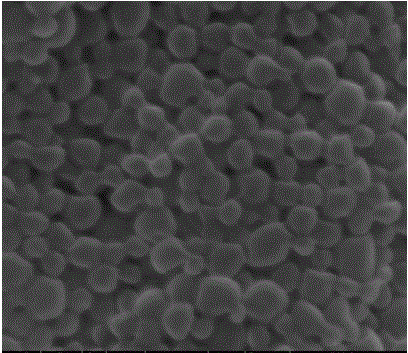
[0049] Laser particle size analysis showed that the obtained microspheres were normally distributed with an effective diameter of 304.3n...
Embodiment 2
[0051] Precisely weigh 10mg10-hydroxycamptothecin bulk drug and 100mgPLGA.
[0052] Dissolve 10-hydroxycamptothecin and PLGA in 5 ml of methanol:dichloromethane (v / v = 1:4) binary solvent. After fully dissolving, the above organic solution was added dropwise to 10ml of a 3% polyvinyl alcohol aqueous solution, and ultrasonically emulsified for 30s under the condition of an amplitude of 60%. Add the emulsified emulsion to 85 ml of polyvinyl alcohol aqueous solution with a mass concentration of 0.3% for dispersion and solidification. Stir at room temperature for 8h to completely evaporate the organic solvent. The cured microspheres were centrifuged at 4° C. at a rotational speed of 10,000 r / min for 10 minutes, collected, and freeze-dried by adding a lyoprotectant to obtain 10-hydroxycamptothecin microsphere powder.
[0053] Laser particle size analysis showed that the obtained microspheres were normally distributed with an effective diameter of 353.7nm, a polydispersity coeffic...
Embodiment 3
[0055] Precisely weigh 20mg10-hydroxycamptothecin bulk drug and 100mgPLGA.
[0056] Dissolve 10-hydroxycamptothecin and PLGA in 5 ml of methanol:dichloromethane (v / v = 1:4) binary solvent. After fully dissolving, the above organic solution was added dropwise to 10ml of a 3% polyvinyl alcohol aqueous solution, and ultrasonically emulsified for 30s under the condition of an amplitude of 38%. Add the emulsified emulsion to 55 ml of polyvinyl alcohol aqueous solution with a mass concentration of 0.3% for dispersion and solidification. Distill under reduced pressure at 10-200 mbar, 30-35°C, and rotary evaporate for 2 hours to completely evaporate the organic solvent. The cured microspheres were centrifuged at 4° C. at a rotational speed of 10,000 r / min for 10 minutes, collected, and freeze-dried by adding a lyoprotectant to obtain 10-hydroxycamptothecin microsphere powder.
[0057] Laser particle size analysis showed that the obtained microspheres were normally distributed with a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com