Acyclic nucleoside cyclic phosphate derivatives, preparation method and medical application thereof
A pharmaceutical and aromatic ring technology, applied in the field of preparation of antiviral drugs, can solve the problems of nephrotoxicity, highly sensitive hydrolysis reaction, no phase 3 clinical reports, etc., and achieve the effect of reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
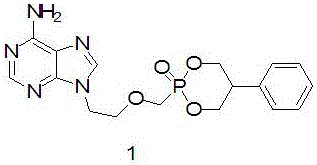
[0036] Preparation of 9-[2-(2-phenyl)-2-oxa-1,3,2-dioxaphosphorylhexyl-2-methyleneoxyethyl]adenine (Compound 1).
[0037]
[0038] N 2 Under protection, suspend PMEA (3.00g, 10.99mmol) in dry dichloromethane (40ml), then add N,N-diethylformamide (1.35ml, 12.12mmol), stir, and slowly add oxalyl chloride dropwise (3.5ml, 36.80mmol), after the dropwise addition, heat and reflux for 2 hours, cool to room temperature, concentrate under reduced pressure, add dry dichloromethane (40ml) to the residue, dissolve and then concentrate under reduced pressure, then add dry dichloromethane (40ml), slowly added pyridine (1.08ml, 13.42mmol) under ice-bath conditions and stored under these conditions for later use.
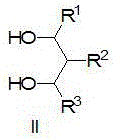
[0039] 2-Phenyl-1,3-propanediol (1.672g, 10.97mmol), triethylamine (7.5ml, 53.96mmol), dissolved in dry dichloromethane (30ml), liquid nitrogen-acetone bath, the reaction solution was cooled to -78°C, then slowly add the above standby solution dropwise, and control the temper...
Embodiment 2
[0042] 9-[2-(1,3-diphenyl)-2-oxa-1,3,2-dioxaphosphorylhexyl-2-methyleneoxyethyl]adenine (compound 2) preparation.
[0043]
[0044] According to the operation of Example 1, PMEA (3.00g, 10.99mmol) and 1,3-diphenylpropanediol (2.51g, 10.99mmol) were reacted as raw materials to generate compound 2, the product weighed 2.29g, and the total yield was 44.8%. MS(ESI), foundm / z466, 488, calcd466[M+H] + , 488[M+Na] + . 1 HNMR (CDCl 3 ): δ=2.10-2.52(m, 2H), 3.88-4.14(m, 4H), 4.28-4.59(t, 2H), 5.42-5.85(m, 2H), 6.29-6.85(s, 2H), 7.10 -7.25 (m, 2H), 7.28-7.50 (m, 8H), 7.80-8.02 (s, 1H), 8.11-8.35 (s, 1H). IR (KBr): 3328, 3162, 1659, 1597, 1247, 1052.
Embodiment 3
[0046] Preparation of 9-[2-(2-benzyloxy)-2-oxa-1,3,2-dioxaphosphorylhexyl-2-methyleneoxyethyl]adenine (Compound 3).
[0047]
[0048] According to the operation of Example 1, PMEA (3.00g, 10.99mmol) and 2-benzyloxy-1,3-propanediol (2.00g, 10.99mmol) were reacted as raw materials to generate compound 3, the product weighed 1.57g, and the total yield was 34.1 %. MS(ESI), foundm / z420, 442, calcd420[M+H] + , 442[M+Na] + . 1 HNMR (CDCl 3 ): δ=3.44-3.52(m, 1H), 3.92-3.98(m, 4H), 4.12-4.37(m, 4H), 4.38-4.45(t, 2H), 4.57-4.64(s, 2H), 5.68 -6.00 (s, 2H), 7.30-7.41 (m, 5H), 7.83-7.94 (s, 1H), 8.28-8.37 (s, 1H). IR (KBr): 3345, 3168, 1650, 1597, 1251, 1048.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com