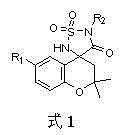
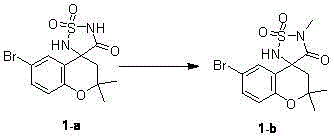Benzo dihydropyran sulfamide spiro-compound and preparation method thereof
A technology of chroman-like sulfonamide spiro and chroman, which is applied in the field of chroman-like sulfonamide spiro compounds and their preparation, and can solve the problem of undiscovered sulfonamide spiro compounds, etc. problem, to achieve the effect of increasing diversity and improving polarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 : 6-Bromo-2,2-dimethylspiro[chroman-4-3'-(1,2,5)thiodiazoline]-4'-one-S,S-di Oxide I-a preparation of
[0043]
[0044] Steps:
[0045] 1-(5-Bromo-2-hydroxyphenyl)ethanone 1 (570 g, 2.6 moles) was dissolved in a mixed solution of 970 milliliters of acetone (13 moles) and 5.5 liters of toluene, then 220 milliliters of tetrahydropyrrole (2.6 moles) was added to the mixed solution and stirred at 80°C for 16 hours. The reaction mixture was concentrated into a black oily crude product, which was purified by column chromatography to obtain 544 g of light yellow oily 6-bromo-2,2-dimethylchroman-4-one 2 , the yield is 80%. HNMR (CDCl 3) d: 7.84 (s, 1H), 7.42 (d, J = 8.8 Hz, 1H), 6.73 (d, J = 8.8 Hz, 1H), 2.61 (s, 2H), 1.32 (s, 6H).
[0046] 200 grams of 6-bromo-2,2-dimethylchroman-4-one 2 (0.78 moles), 550 grams of ammonium carbonate (5.5 moles), 104 grams of potassium cyanide (1.56 moles) and 1.2 liters of ammonium formate were added to the autoclave....
Embodiment 2
[0056] Example 2 : 6-bromo-2,2,5'-trimethylspiro[chroman-4-3'-(1,2,5)thiodiazoline]-4'-one-S, S-dioxide I-b preparation of
[0057]
[0058] Steps:
[0059] 359 mg of 6-bromo-2,2-dimethylspiro[chroman-4-3'-(1,2,5)thiodiazoline]-4'-one-S,S - Dioxide I-a (1 mmol), was added to 50 ml of tetrahydrofuran solution, and then 60 mg of sodium hydride (60% in mineral oil) was added. The mixture was stirred at room temperature 25°C for 0.5 hours, and then 142 mg of methyl iodide was added to the mixture. The reaction solution was stirred at room temperature for 16 hours and then concentrated. The resulting crude product was separated by column chromatography to obtain 340 mg of 6-bromo-2,2,5 '-Trimethylspiro[chroman-4-3'-(1,2,5)thiodiazoline]-4'-one-S,S-dioxide I-b . Yield: 91%.
[0060] HNMR (DMSO) d: 11.2 (br s, 1H), 7.35 (s, 1H), 7.22 (d, J = 8.8 Hz, 1H), 6.95 (br s, 1H), 6.65 (d, J = 8.8 Hz, 1H), 2.71 (s, 3H), 2.41 (d, J = 14.4 Hz, 1H), 1.88 (d, J = 14.4 Hz, 1H)...
Embodiment 3
[0061] Example 3 : 6-bromo-2,2,-trimethyl 5'-benzylspiro[chroman-4-3'-(1,2,5)thiodiazoline]-4'-one -S,S-dioxide 1-c preparation of
[0062]
[0063] Steps:
[0064] 359 mg of 6-bromo-2,2-dimethylspiro[chroman-4-3'-(1,2,5)thiodiazoline]-4'-one-S,S - Dioxide I-a (1 mmol), was added to 50 ml of tetrahydrofuran solution, and then 60 mg of sodium hydride (60% in mineral oil) was added. The mixture was stirred at room temperature 25°C for 0.5 hours, and then 126 mg of benzyl chloride was added to the mixture. The reaction solution was stirred at room temperature for 16 hours and then concentrated. The resulting crude product was separated by column chromatography to obtain 420 mg of 6-bromo-2,2,- Trimethyl 5'-benzylspiro[chroman-4-3'-(1,2,5)thiodiazoline]-4'-one-S,S-dioxide 1-c . Yield: 93%.
[0065] HNMR (DMSO) d: 7.35 (s, 1H), 7.33-7.29 (m, 3H), 7.22 (d, J = 8.8 Hz, 1H), 7.19 (d, J = 7.6 Hz, 2H), 6.95 (br s, 1 H), 6.65 (d, J = 8.8 Hz, 1H), 4.50 (s, 2H), 2.41 (d, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ec50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com