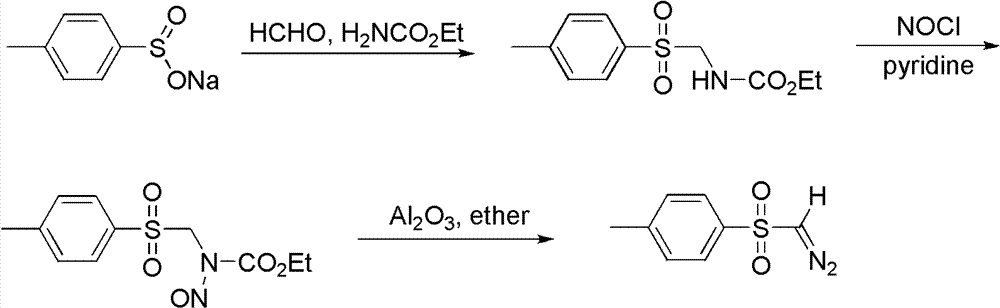
Synthetic method of diazomethane compound
A synthesis method and diazomethane technology are applied in the preparation of organic compounds, organic chemistry methods, chemical instruments and methods, etc., which can solve problems such as environmental hazards and increased operation difficulty, and achieve low energy demand and simple post-processing process. , to avoid the effect of silica gel column chromatography separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The synthesis of embodiment 1 p-toluenesulfonyl acetate-3,3,5-trimethyl-2-cyclohexenol ester
[0041]
[0042] 3,3,5-trimethyl-2-cyclohexenol (6mmol) was dissolved in dichloromethane (10ml), and 4-dimethylaminopyridine (DMAP) (0.6mmol) was added to the reaction system, Further dicyclohexylcarbodiimide (DCC) (6.6 mmol) was dissolved in dichloromethane (6 ml) and added to the solution. After 5 minutes, p-toluenesulfonylacetic acid (6.6 mmol) was added to the reaction system, and the clear liquid immediately became turbid. Stir at room temperature for 12 hours. The solid in the flask was filtered off and the filtered solid was washed with EtOAc (20ml). The filtrate was washed with potassium bisulfate (1M, 20ml×3), saturated sodium bicarbonate (20ml×3), saturated brine (20ml×3), and dried over anhydrous magnesium sulfate. The solvent was concentrated under reduced pressure and purified by silica gel column chromatography (ethyl acetate: petroleum ether) to obtain the...
Embodiment 2
[0043] The synthesis of embodiment 2 p-toluenesulfonyl acetate-2-cyclohexenol ester
[0044] 2-cyclohexenol was dissolved in dichloromethane, DMAP was added to the reaction system, DCC was dissolved in dichloromethane and then added to the solution. After 5 minutes, p-toluenesulfonylacetic acid was added to the reaction system, and the clear liquid immediately became turbid. Stir at room temperature for 12 hours. The solid in the flask was filtered off and the filtered solid was washed with EtOAc (20ml). The filtrate was washed with potassium bisulfate (1M, 20ml×3), saturated sodium bicarbonate (20ml×3), saturated brine (20ml×3), and dried over anhydrous magnesium sulfate. The solvent was concentrated under reduced pressure and purified by silica gel column chromatography (ethyl acetate: petroleum ether) to obtain the obtained product.
Embodiment 3
[0045] The synthesis of embodiment 3 p-toluenesulfonyl acetate allyl alcohol ester
[0046] Allyl alcohol was dissolved in dichloromethane, DMAP was added to the reaction system, DCC was dissolved in dichloromethane and then added to the solution. After 5 minutes, p-toluenesulfonylacetic acid was added to the reaction system, and the clear liquid immediately became turbid. Stir at room temperature for 12 hours. The solid in the flask was filtered off and the filtered solid was washed with EtOAc (20ml). The filtrate was washed with potassium bisulfate (1M, 20ml×3), saturated sodium bicarbonate (20ml×3), saturated brine (20ml×3), and dried over anhydrous magnesium sulfate. The solvent was concentrated under reduced pressure and purified by silica gel column chromatography (ethyl acetate: petroleum ether) to obtain the obtained product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com