Lithium ferric manganese phosphate as cathode material of nanometer fibrous lithium ion battery and preparation method of lithium ferric manganese phosphate
A lithium-ion battery, lithium iron manganese phosphate technology, applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems of low discharge voltage platform, poor rate performance and specific capacity density, poor cycle performance of lithium manganese phosphate materials, etc. Achieve the effect of low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
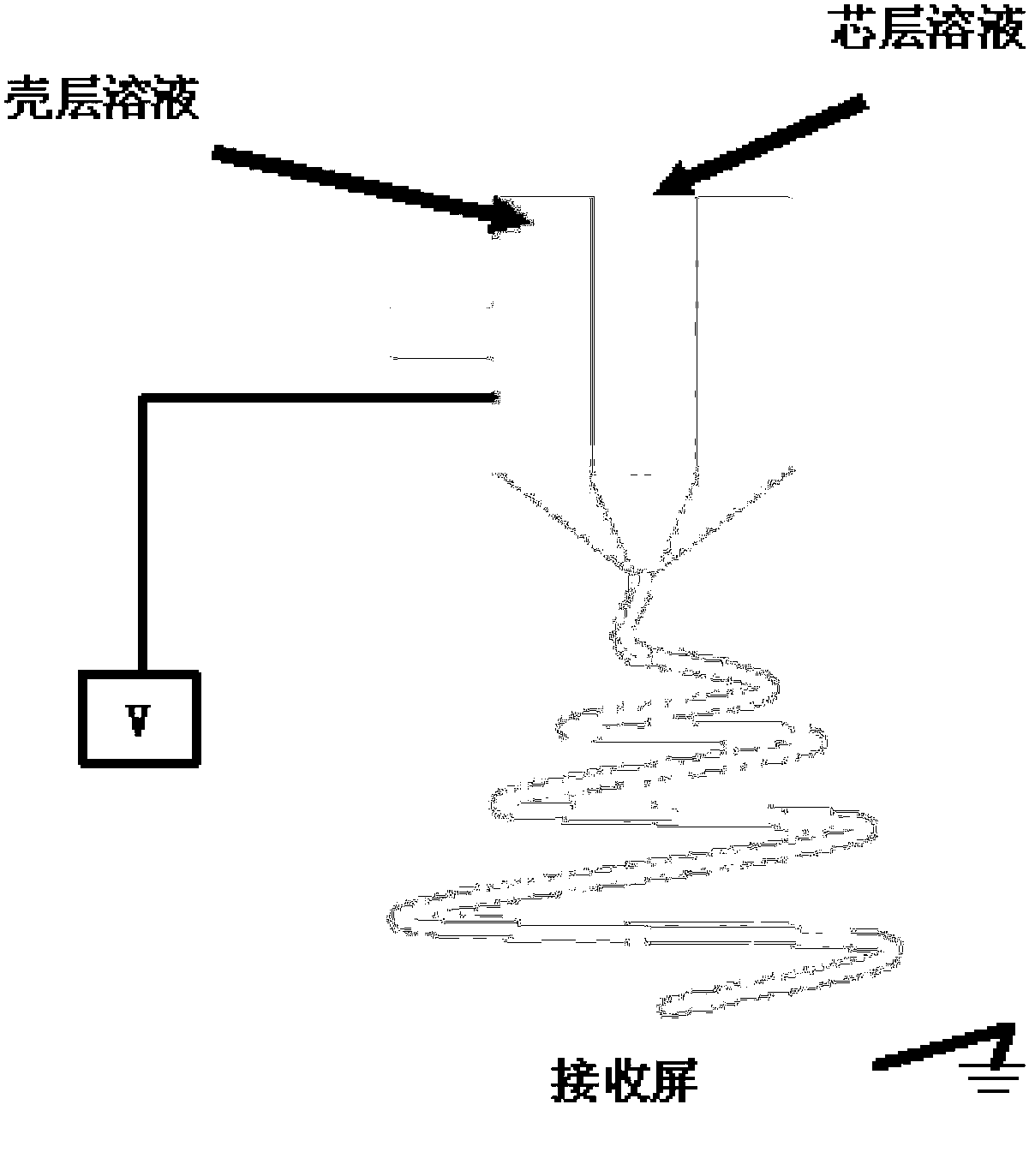
[0026] At a temperature of 20°C and a humidity of 50%, weigh 0.9g of polyvinylidene fluoride and dissolve it in 3mL of acetone and 7mL of a volatile solvent, then add 0.179g of ferrous acetate (Fe(Ac) 2 ), 0.115g of 85wt% phosphoric acid (H 3 PO 4 ) and 0.068g of lithium acetate (LiAc), evenly dispersed and put into the shell syringe; Weigh 0.8g of polyvinylidene fluoride and dissolve in 3mL of acetone and 7mL of volatile solvent, then add 0.245g of manganese acetate tetrahydrate (Mn(Ac) 2 4H 2 O), 0.115g of 85wt% phosphoric acid (H 3 PO 4 ) and 0.068g of Lithium Acetate (LiAc), evenly dispersed into the core syringe; the above-mentioned volatile solvents are ethanol, acetone, chloroform, dichloromethane, N, N-dimethylformamide or N-methyl One or more of pyrrolidone; the electric field intensity is controlled at 0.8KV / cm, the advancing speed of the shell injector is 0.15mL / h, and the advancing speed of the core injector is 0.05mL / h for electrospinning (see figure 1 ); af...
Embodiment 2
[0030] In an environment with a temperature of 25°C and a humidity of 20%, weigh 1.8g of polyacrylonitrile and dissolve it in 20mL of N,N-dimethylformamide, then add 0.160g of ferric oxide (Fe 2 o 3), 0.231g of ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ) and 0.079g of lithium carbonate (Li 2 CO 3 ), and put it into a shell syringe after dispersing evenly; weigh 1.7g of polyvinylidene fluoride and dissolve it in 20mL of N,N-dimethylformamide, and then add 0.490g of manganese acetate tetrahydrate (Mn(Ac) 2 4H 2 O), 0.231g of ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ) and 0.079g of lithium carbonate (Li 2 CO 3 ), dispersed evenly and put into the core injector; the electric field intensity was controlled at 1.0KV / cm, the advancing speed of the shell injector was 0.18mL / h, and the advancing speed of the core injector was 0.07mL / h for electrospinning; After the electrospinning product was dried, it was reacted in a nitrogen atmosphere at 650°C for 8 hours, and the...
Embodiment 3
[0032] In an environment with a temperature of 20°C and a humidity of 70%, weigh 2.7g of polyvinylpyrrolidone and dissolve it in 30mL of ethanol solvent, then add 0.699g of ferric oxalate pentahydrate (Fe 2 (C 2 o 4 ) 3 ·5H 2 O), 0.396g of diammonium hydrogen phosphate ((NH 3 ) 2 HPO 4 ) and 0.072g of lithium hydroxide (LiOH), evenly dispersed and put into the shell syringe; Weigh 2.7g of polyvinylpyrrolidone and dissolve it in 30mL of ethanol, then add 0.735g of manganese acetate tetrahydrate (Mn(Ac) 2 4H 2 O), 0.398g of diammonium hydrogen phosphate ((NH 3 ) 2 HPO 4 ) and 0.072g of Lithium Hydroxide (LiOH), evenly dispersed and put into the core injector; the electric field intensity is controlled at 1.8KV / cm, the advancing speed of the shell injector is 0.21mL / h, and the advancing speed of the core injector is 0.07 mL / h for electrospinning (see equipment schematic diagram figure 1 ); dry the obtained electrospinning product and react it in a nitrogen atmosphere a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com