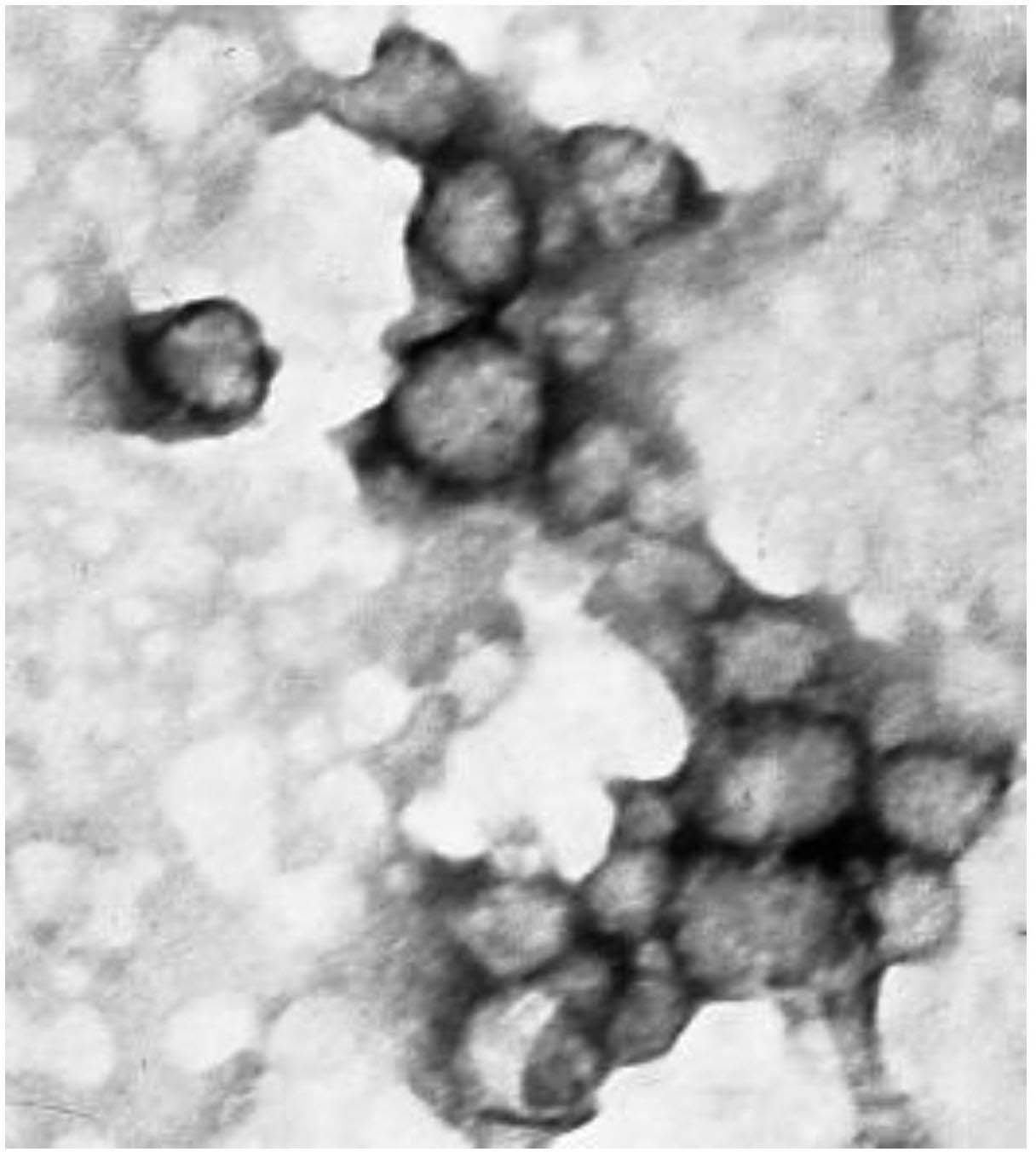
Application of ursolic-acid nano medicine-carrying microspheres for treating tumors and preparation
A nano-loaded drug and ursolic acid technology, which is applied in the direction of antineoplastic drugs, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of limited application, easy formation of embolism, high cost, etc., and achieve cycle time extension and preparation The method is simple and easy, and the effect of superior anti-tumor drug effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Step 1, dispensing: take by weighing 60g ursolic acid and 940g drug-loaded material respectively according to the weight ratio of raw material components, wherein, the drug-loaded material is the amphiphilic block copolymer polymer polycaprolactone and polyethylene glycol synthesis. Caprolactone-polyethylene glycol (mPEG-PCL), wherein: mPEG molecular weight 4000, PCL molecular weight 20000;
[0041] Step 2, drug dissolving: under normal temperature conditions, raw material components such as 60g ursolic acid and 940g drug-loaded material taken in step 1 are dissolved in a certain amount of acetone solvent (by weight ratio of components, raw material group points 80%, acetone solvent 20%);
[0042] Step 3, dilution: Slowly add the acetone solution obtained in step 2 to a certain amount of distilled water under stirring conditions (the volume ratio of acetone solution to distilled water is 1:4), and then obtain light blue ursolic acid microspheres acetone and water mixed...
Embodiment 2
[0047] Step 1, dispensing: weigh 200g ursolic acid and 800g drug-loaded material according to the weight ratio of the raw material components, wherein the drug-loaded material is an amphiphilic block copolymer polylactic acid- Polyethylene glycol (mPEG-PLA), in which: mPEG molecular weight 4000, PLA molecular weight 40000;
[0048] Step 2, drug dissolving: under normal temperature conditions, raw material components such as 200g ursolic acid and 800g drug-loaded material taken in step 1 are dissolved in a certain amount of acetone solvent (by weight ratio of components, raw material group 65%, acetone solvent 35%);
[0049] Step 3, dilution: slowly drop the acetone solution obtained in step 2 into a certain amount of distilled water under stirring conditions (the volume ratio of acetone solution to distilled water is 1:4.5), and then obtain light blue ursolic acid microspheres acetone and water mixed solution;
[0050] Step 4, purification: put the diluted acetone solution o...
Embodiment 3
[0055] Step 1, dispensing: Weigh 360g ursolic acid and 640g drug-loaded material according to the weight ratio of the raw material components, wherein the drug-loaded material is an amphiphilic block copolymer polyhydroxy Acetic acid-polyethylene glycol (mPEG-PLGA), in which: mPEG molecular weight is 4000, PLGA molecular weight is 36000;
[0056] Step 2, drug dissolving: under normal temperature conditions, raw material components such as 360g ursolic acid and 640g drug-loaded material taken in step 1 are dissolved in a certain amount of acetone solvent (by weight ratio of components, raw material group points 50%, acetone solvent 50%);
[0057] Step 3, dilution: Slowly add the acetone solution obtained in step 2 to a certain amount of distilled water under stirring conditions (the volume ratio of acetone solution to distilled water is 1:5), and then obtain light blue ursolic acid microspheres acetone and water mixed solution;
[0058] Step 4, purification: put the diluted a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com