Preparation method of aspirin enteric-coated sustained-release preparation
A technology for aspirin and sustained-release preparations, which is applied in the field of preparation of enteric-coated sustained-release preparations, can solve the problems of decreased drug dissolution, more enteric-coated materials, and increased hardness, and achieves reduced dosage, smooth release, and compliance. improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] prescription
[0049] ⑴ Chip core
[0050]
[0051] ⑵Enteric coating layer
[0052]
[0053] craft
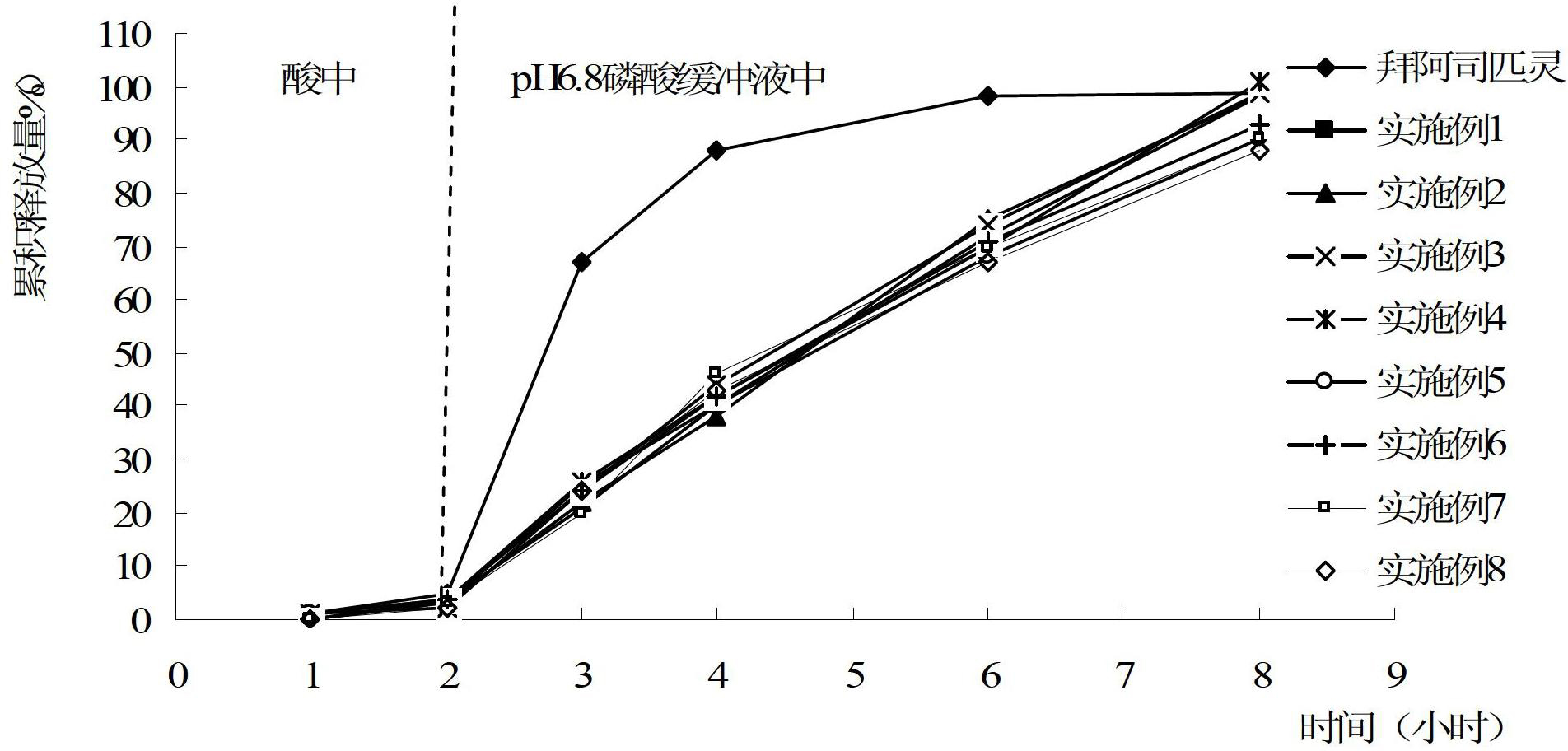
[0054] (1) Take aspirin for tablet core, add hypromellose K15M, microcrystalline cellulose PH102, lactose and sodium lauryl sulfate, and mix well. Put it in a rotary tablet press (C&C800, Beijing Chuangbo Jiawei Technology Co., Ltd., the same below), use a shallow concave circular punching tablet with a diameter of 6mm, adjust the tablet weight and pressure, control the hardness to 50-80N, and prepare a total of 1000 pieces.
[0055] (2) For the enteric coating layer, take Eudragit RS 30D and Eudragit RL 30D aqueous dispersions (both are products of Rohm, Germany), add triethyl citrate talcum powder, 95% ethanol (v / v) and water, Stir well. Put the above-mentioned tablets in a coating machine (BGB-5B type, Wenzhou Pharmaceutical Equipment Factory, the same below), adjust the coating machine speed, liquid spray speed, inlet air temperature, inlet air pressure, a...
Embodiment 2
[0058] prescription
[0059] ⑴ Chip core
[0060]
[0061] ⑵Isolation gown layer
[0062] Hypromellose 17g
[0063] Macrogol 400 3g
[0064] 60% ethanol solution (v / v) 280g
[0065] ⑶ enteric coating layer
[0066]
[0067] craft
[0068] (1) Take aspirin for tablet core, add hypromellose K4M, microcrystalline cellulose PH102, pregelatinized starch and sodium lauryl sulfate, and mix well. Put it in a rotary tablet press, adopt a shallow concave circular punching tablet with a diameter of 6.5mm, adjust the tablet weight and pressure, control the hardness to 60-100N, and prepare 1000 tablets in total.
[0069] (2) For the isolation layer, dissolve hypromellose and polyethylene glycol 400 in 60% ethanol solution and stir to dissolve completely. The above-mentioned tablets are placed in the coating machine, and the rotating speed of the coating machine, the liquid spray speed, the air inlet temperature, the air inlet air pressure, the atomization pressure, and the spr...
Embodiment 3
[0072] prescription
[0073] ⑴ Chip core
[0074]
[0075] ⑵Enteric coating layer
[0076]
[0077] craft
[0078] (1) Take aspirin for tablet core, add hydroxymethylcellulose Klucel HF (manufactured by Aqualon), tartaric acid, and dextrin, and mix well. Put it in a rotary tablet press, adopt a shallow concave circular punching tablet with a diameter of 6.5mm, adjust the tablet weight and pressure, control the hardness to 60-100N, and prepare 1000 tablets in total.
[0079] (2) Take acetone for the enteric coating layer, add hypromellose phthalate, triacetin and glycerin, stir well, then add water and talcum powder, mix well. The above-mentioned tablets are placed in the coating machine, and the rotating speed of the coating machine, the liquid spray speed, the air inlet temperature, the air inlet air pressure, the atomization pressure, and the spray distance of the tablet are adjusted, and the temperature of the tablet is controlled to be 35-43° C. The weight is abo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com