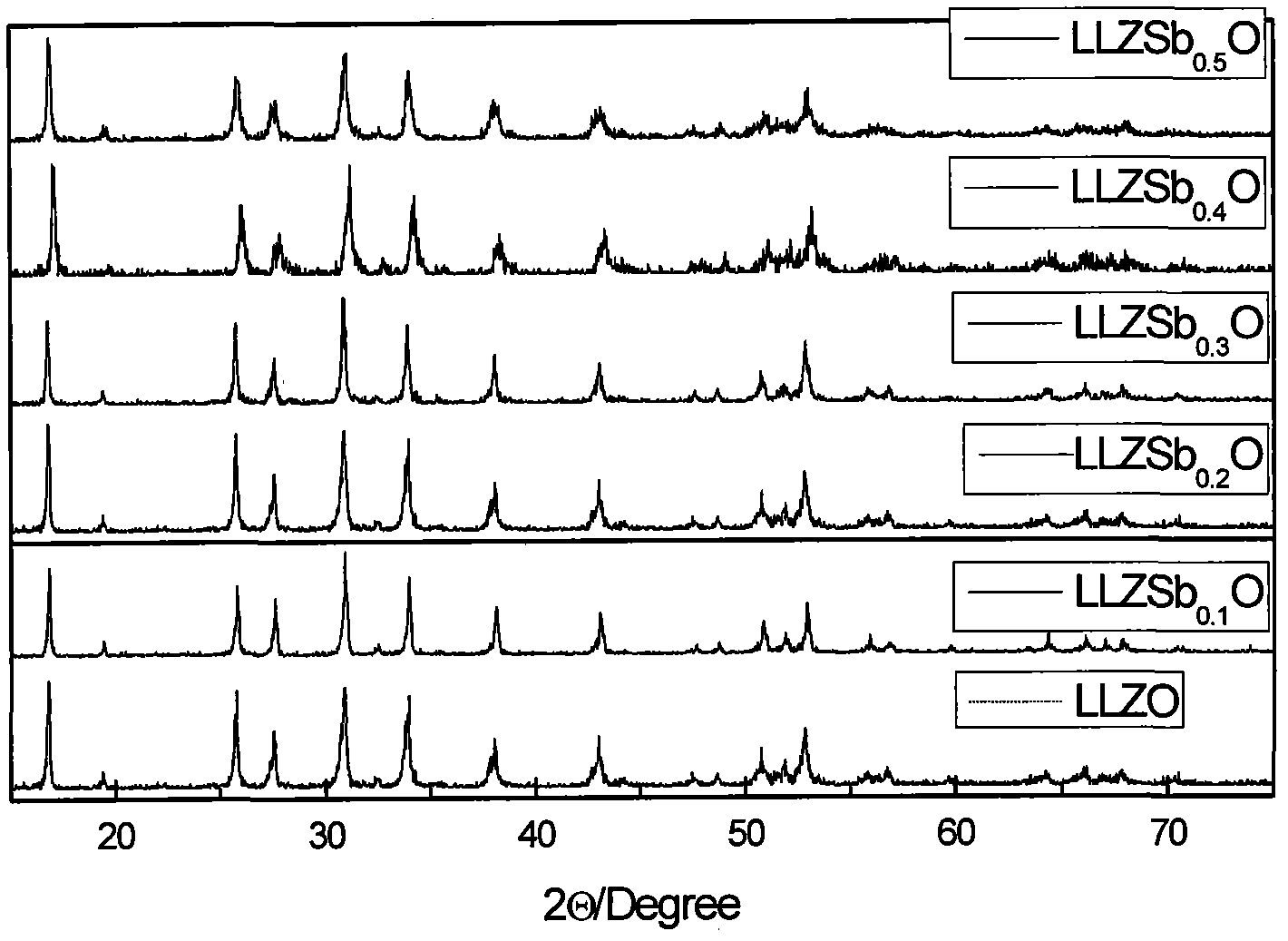
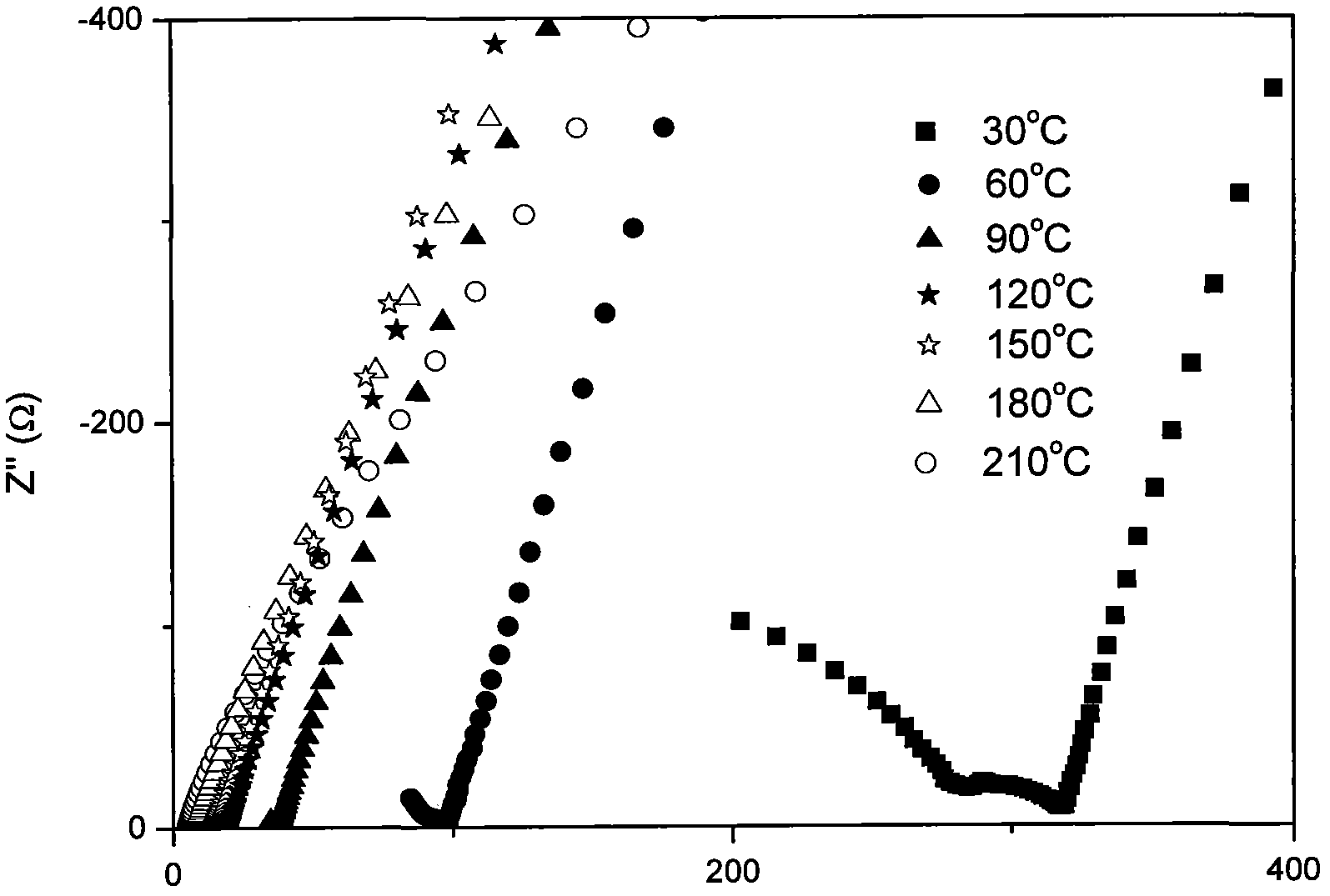
Stibonium-doped quasi garnet-structured lithium ion crystalline-state solid electrolyte material and synthesis method thereof
A technology of solid electrolyte and synthesis method, which is applied in the field of lithium ion crystalline ceramic solid electrolyte material and its synthesis, can solve the problems of delaying the application process of lithium ion solid electrolyte, prolonging the process route, and low synthesis temperature, so as to increase lithium ion vacancies , Improve the conductivity and reduce the effect of sintering temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Li 6.9 La 3 Zr 1.9 Sb 0.1 o 12 Preparation of raw material powder required for synthesis: lanthanum oxide (La 2 o 3 ) was baked at 900°C for 6 hours, lithium carbonate (Li 2 CO 3 ), zirconia (ZrO 2 ) and antimony trioxide (Sb 2 o 3 ) were dried at 120°C for 5 hours.
[0033] (2) Weighing and batching of raw material powder: the raw material powder that synthetic 5g needs to weigh is weighed respectively by the lanthanum oxide (La) prepared in the step (1) by stoichiometric ratio 2 o 3 99.99%) 2.9020g, lithium carbonate (Li 2 CO 3 98%) 1.5445g, Zirconia (ZrO 2 99.9%) 1.3915g and antimony trioxide (Sb 2 o 3 99%) 0.0874g, wherein lithium carbonate adds 10% more in order to compensate the lithium lost in high temperature. Mix the accurately weighed powder in an agate mortar, add 10ml of distilled water as an abrasive, mix and grind thoroughly to make the powder uniform.
[0034] (3) Li 6.9 La 3 Zr 1.9 Sb 0.1 o 12 Synthesis of the composite: the ra...
Embodiment 2
[0038] (1) Li 6.8 La 3 Zr 1.8 Sb 0.2 o 12 Preparation of raw material powder required for synthesis: lanthanum oxide (La 2 o 3 ) was baked at 900°C for 7 hours, lithium carbonate (Li 2 CO 3 ), zirconia (ZrO 2 ) and antimony trioxide (Sb 2 o 3 ) were dried at 120°C for 7 hours.
[0039] (2) Weighing and batching of raw material powder: the required weighing raw material powder of synthetic 7g is weighed respectively by the lanthanum oxide (La) prepared in step (1) by stoichiometric ratio 2 o 3 99.99%) 4.0515g, lithium carbonate (Li 2 CO 3 98%) 2.1250g, Zirconia (ZrO 2 99.9%) 1.8404g and antimony trioxide (Sb 2 o 3 99%) 0.2440, wherein lithium carbonate is added 10% more in order to compensate the lithium lost in high temperature. Mix the accurately weighed powder in an agate mortar, add 15ml of distilled water as an abrasive, mix and grind thoroughly to make the powder uniform.
[0040] (3) Li 6.8 La 3 Zr 1.8 Sb 0.2 o 12 Synthesis of the composite: the ra...
Embodiment 3
[0044] (1) Li 6.7 La 3 Zr 1.7 Sb 0.3 o 12 Preparation of raw material powder required for synthesis: lanthanum oxide (La 2 o 3 ) was calcined at 900°C for 9 hours, lithium carbonate (Li 2 CO 3 ), zirconia (ZrO 2 ) and antimony trioxide (Sb 2 o 3 ) were dried at 120° C. for 9 hours, respectively.
[0045] (2) Weighing and batching of raw material powder: the raw material powder that synthesizes 10g required weighing is weighed respectively by the lanthanum oxide (La) prepared in step (1) by stoichiometric ratio 2 o 3 99.99%) 5.8041g, lithium carbonate (Li 2 CO 3 98%) 2.9995g, Zirconia (ZrO 2 99.9%) 2.4901g and antimony trioxide (Sb 2 o 3 99%) 0.5245g, wherein lithium carbonate adds 10% more in order to compensate the lithium lost in high temperature. Mix the accurately weighed powder in an agate mortar, and add 20ml of absolute ethanol as a grinding agent, mix and grind thoroughly to make the powder uniform.
[0046] (3) Li 6.7 La 3 Zr 1.7 Sb 0.3 o 12 Syn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com