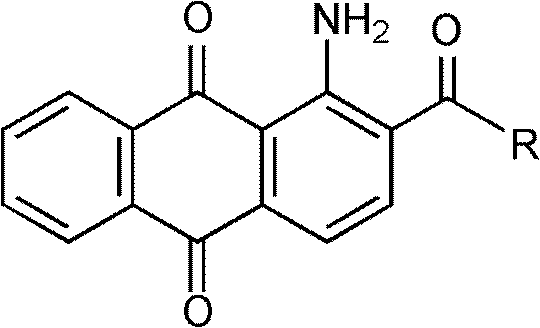
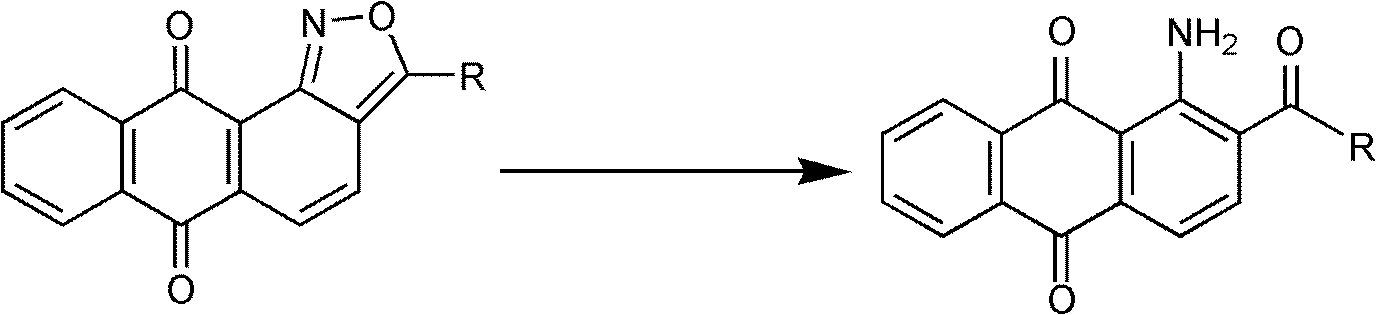
New synthesis process of 1-amino-2-acetylanthraquinone and derivatives thereof
A technology for the synthesis of acetyl anthracene, which is applied in the field of new synthesis technology of 1-amino-2-acetyl anthraquinone and its derivatives, can solve the problems of heavy pollution of three wastes and low yield, and achieve cheap and high yield. High efficiency and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 30ml of water, 0.2-0.8g of ferric chloride and 6g of raw material 3-methyl-anthraquinone-[1,2-c]-isoxazole to a 250ml four-necked flask, raise the temperature to 20-100°C, and stir for 0.5 h, add 10-30ml of 40-80% hydrazine hydrate dropwise at the temperature, keep the temperature for 4-12h, filter with suction, wash the filter cake with water, and dry to obtain the product, weighing 5.2g, with a yield of 86.0%.
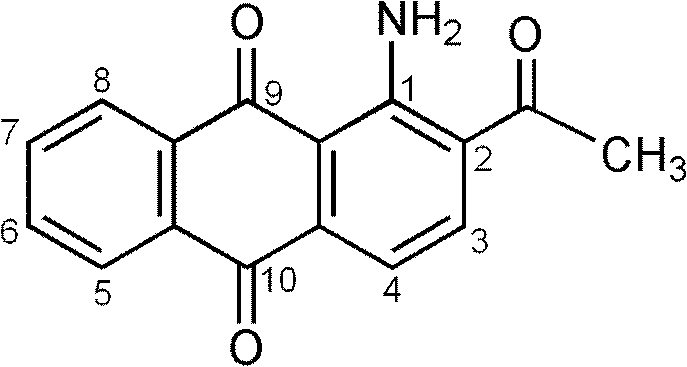
[0027] Product structure through mass spectrometry (mass spectrometer: HP 1100LC-MSD) and 1 HNMR (Nuclear Magnetic Resonance Apparatus: Varian INOVA400M NMR) measurement and confirmation: the synthesized product is dissolved in chloroform, and the APCI ion source is used for mass spectrometry analysis. The molecular ion peak (M+1) is: e / m=266; the product is deuterated After the chloroform was dissolved, the 1 HNMR analysis, the data is: δ2.68 (3H, s, CH 3 ), δ7.55(1H, d, H 3 ), δ7.72-7.83 (2H, t, H 6,7 ), δ8.16(1H, d, H 4 ), δ8.23-8.32 (2H, d, H 5,8...
Embodiment 2
[0031] Add 30ml of water and 6g of raw material 3-propyl-anthraquinone-[1,2-c]-isoxazole to a 250ml four-neck flask, raise the temperature to 20-100°C, stir for 0.5h, and dropwise add 40-80% hydrazine hydrate 10-30ml, heat preservation reaction for 4-12h, suction filtration, filter cake washed with water, dried to obtain the product, weighing 5.1g, yield 84.4%. The product structure adopts the mass spectrometer and the nuclear magnetic resonance instrument in the embodiment 1 to measure and confirm.
Embodiment 3
[0033] Add 20-160mL water, 6g raw material 3-methyl-anthraquinone-[1,2-c]-isoxazole, 3-15g iron powder into a 250ml four-neck flask, and reflux at 100°C for 3-10h. After the reaction, iron sludge was removed, and the filter cake was washed with water and dried to obtain the product, weighing 6g, with a yield of 99.2%. The product structure adopts the mass spectrometer and the nuclear magnetic resonance instrument in the embodiment 1 to measure and confirm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com