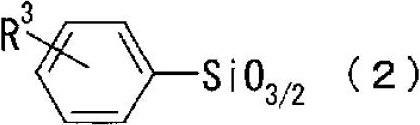Photosensitive Resin Composition
A technology of photosensitive resin and composition, which is applied in optics, optomechanical equipment, instruments, etc., can solve the problem of charge mobility decline, and achieve the effect of excellent resistance to change over time and high transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example A
[0092] [Production Example A: Production of Polysiloxane Compound A]
[0093] Add 124.2 g (0.5 moles) of 3-methacryloxypropyltrimethoxysilane, 39.7 g (0.2 moles) of phenyltrimethoxysilane, dimethyl 132.2 g (1.1 mol) of dimethoxysilane, 75.3 g (0.2 mol) of diphenyldimethoxysilane, and 300 g of 1-butanol as a solvent. After stirring and heating to 70° C., 100 parts of 0.12% phosphoric acid aqueous solution was added dropwise, and then reacted at 80° C. for 1 hour. Next, an aqueous sodium hydroxide solution was added to neutralize the reaction liquid, followed by reaction at 80° C. for 1 hour. After adding 300 g of toluene, stirring was stopped, and the separated water-containing layer was removed. After washing the toluene layer with 1000 g of water three times, the solvent was distilled off under reduced pressure at 40° C. under a nitrogen stream. 300 g of triethyl orthoformate was added thereto, and after treatment at 130° C. for 1 hour, volatile components such as unreacte...
manufacture example B~I
[0094] [Production Examples B to I: Production of Polysiloxane Compounds B to I]
[0095] In manufacture example A, except having used the alkoxysilane compound shown in Table 1, operation similar to manufacture example A was performed, and polysiloxane compound B-I shown in Table 1 was synthesize|combined. The numerical values in the table represent the reaction molar ratios of the alkoxysilane compounds. Among the polysiloxane compounds in Table 1, polysiloxane compounds A to E are polysiloxane compounds of the present invention, and polysiloxane compounds F to I are comparative polysiloxane compounds. In addition, Table 2 shows the analysis results of the mass average molecular weight and the silanol group content.
[0096] Table 1
[0097] polysiloxane compound
A
B
C
D
E
F
G
H
I
3-Methacryloxypropyltrimethoxysilane
25
25
25
15
15
-
-
-
-
Ph...
Embodiment 1~7 and comparative example 1~5
[0101] Using polysiloxane compounds A to I, the following acrylates A and B, and bis(2,6-dimethoxybenzoyl)phenylphosphine oxide (hereinafter referred to as photo Radical generator 1), 1-hydroxycyclohexyl phenyl ketone (hereinafter referred to as photoradical generator 2), [4-(2-chloro-4-benzoylphenylthio) as a photoacid generator Phenylbis(4-chlorophenyl)sulfonium hexafluoroantimonate], platinum-divinyltetramethyldisiloxane complex as hydrosilylation catalyst, and butyl acetate as solvent, according to the table Composition of 3. The photosensitive resin composition or thermosetting resin composition of Examples 1-7 and Comparative Examples 1-5 were prepared.
[0102]
[0103] Acrylate A
[0104]
[0105] Acrylate B
[0106] table 3
[0107]
[0108]
[0109] In order to evaluate the characteristics of the photosensitive resin composition or thermosetting resin composition obtained in the above-mentioned examples and comparative examples as a gate insulating film...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com