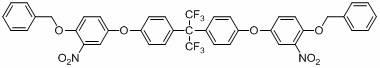
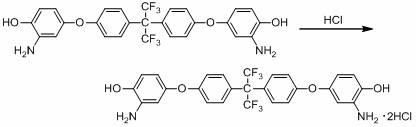
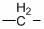Bisphenol 2 (m-amino p-hydroxy phenyl) ether hydrochloride and preparation method and application thereof
A technology of nitro-p-hydroxyhalobenzene and hydroxyphenoxy is applied in the field of benzoxazole-based polymer monomers and their preparation, and can solve the problems of poor practicability, increased cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0215] Step Ⅰ: Preparation of m-nitro-p-hydroxyhalobenzene (Example 1)
[0216] Embodiment 1: Synthesis of 3-nitro-4-hydroxychlorobenzene
[0217] Put 152.62g (1.188mol) of p-hydroxychlorobenzene in the reaction bottle, add 1500ml of dichloromethane to dissolve, cool to 0°C under stirring, add 126.74g of concentrated nitric acid (65%, 1.308mol) dropwise, and control the temperature below 5°C , after the dropwise addition was completed, the temperature was controlled below 5°C and stirred for 3 hours. 1500ml of water was washed twice, 500ml of methanol was added, dichloromethane was distilled off, and 180.51g of pure 3-nitro-4-hydroxychlorobenzene was obtained by suction filtration, a yellow powder with a yield of 87.6%.
[0218] The structure is characterized as follows: Infrared spectrum (KBr, cm -1 ) 3421, 3064, 1537, 1473, 1335, 1291, 1222, 818; elemental analysis (%): theoretical value: C: 41.52, H: 2.32, N: 8.07; measured value: C: 41.57, H: 2.30, N :8.12; 1 HNMR: (CD...
Embodiment 2
[0220] Embodiment 2: Synthesis of 3-nitro-4-benzyloxychlorobenzene
[0221] 126.34 g (0.726 mol) of 3-nitro-4-hydroxychlorobenzene was placed in a reaction flask, and 1000 ml of acetonitrile was added. After dissolution, 124.58 g (0.903 mol) of potassium carbonate, 92.46 g of benzyl chloride (0.730 mol) and 10.39 g of potassium iodide (0.0626 mol) were added under stirring, and heated to reflux for 2 hours. After cooling, distill off acetonitrile, add 1000ml of water, stir for 45min, filter with suction, and wash twice with 1000ml of water to obtain a crude product. The crude product was recrystallized from ethanol to obtain 178.24 g of pure 3-nitro-4-benzyloxychlorobenzene as yellow crystals, with a yield of 93.0%.
[0222] The structure is characterized as follows: Infrared spectrum (KBr, cm -1 ) 3092, 3029, 2866, 1611, 1567, 1529, 1481, 1454, 1342, 1301, 1264, 1225, 1132, 1018, 859, 814, 747, 706; elemental analysis (%): theoretical value: C: 59.22, H: 3.82, N: 5.31; mea...
Embodiment 3
[0224] Example 3: Synthesis of 2,2-bis(4-(3-nitro-4-benzyloxyphenoxy)phenyl)propane
[0225] 30.90g (0.117mol) of 3-nitro-4-benzyloxychlorobenzene, 13.33g (0.0585mol) of bisphenol A, 17.14g (0.124mol) of potassium carbonate, and 1.17g (0.0118mol) of cuprous chloride Put it in a reaction flask, and add 150ml of N-methylpyrrolidone. Stir under the protection of nitrogen, and heat to 150 ° C for 18 hours. After cooling, pour it into 1200ml of water, stir for 10 minutes, then filter with suction, wash twice with 400ml of water to obtain a crude product. The crude product was recrystallized with a mixed solvent of ethyl acetate and n-hexane to obtain 28.61g of pure 2,2-bis(4-(3-nitro-4-benzyloxyphenoxy)phenyl)propane, yellow powder , yield 71.6%.
[0226] The structure is characterized as follows: Infrared spectrum (KBr, cm -1 ) 3074, 2820, 1568, 1534, 1471, 1361, 1255, 1139, 1016, 852, 803, 759, 714; element analysis (%): theoretical value: C: 72.13, H: 5.02, N: 4.10; measured...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| modulus | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com