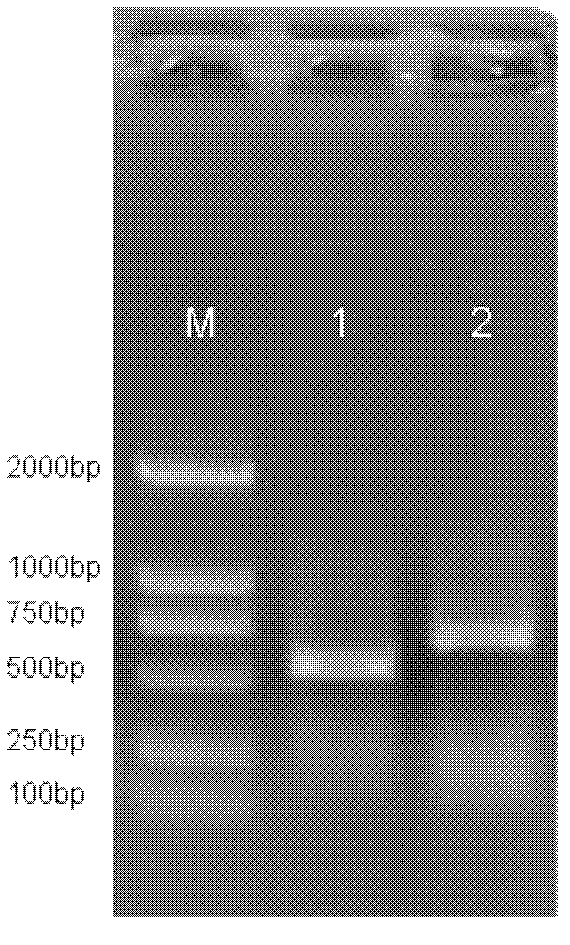Human-mouse chimeric monoclonal antibody against human platelet membrane glycoprotein Ib alpha and applications of human-mouse chimeric monoclonal antibody
A monoclonal antibody and membrane glycoprotein technology, applied in the biological field, can solve problems such as limiting the evaluation of antibody druggability, weakening, and increasing the probability of immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
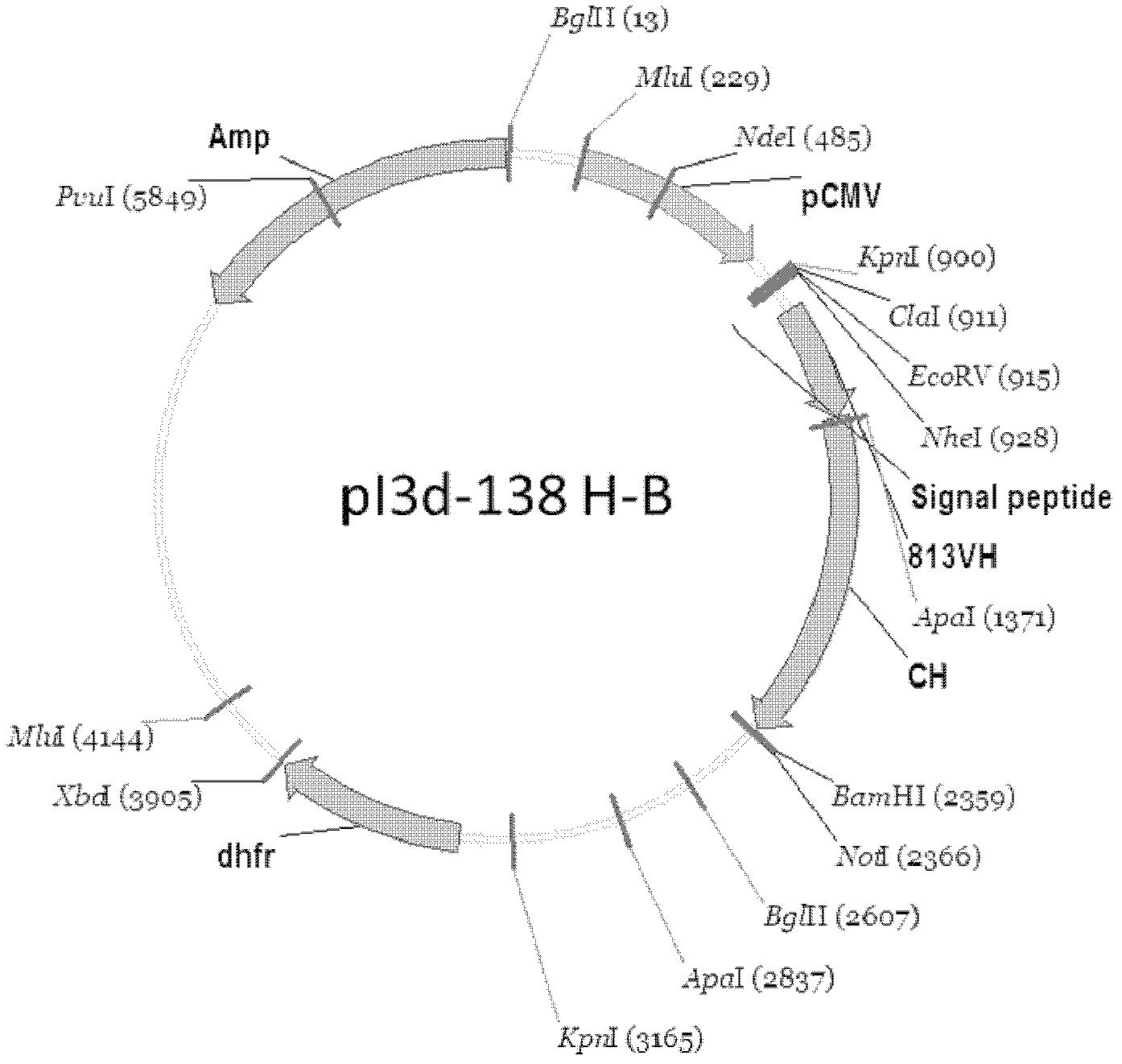
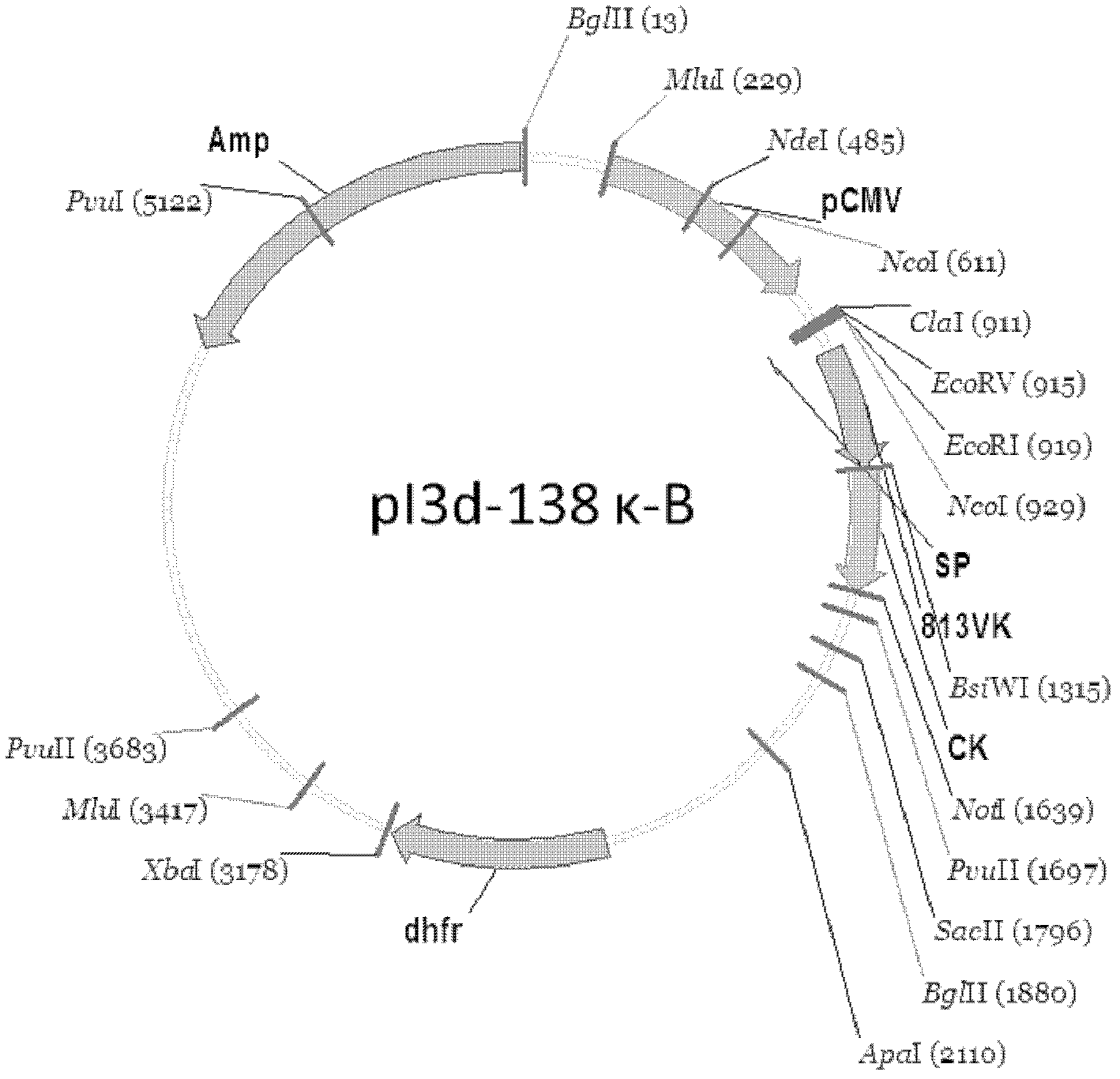
[0053] Example 1: Construction of Chimeric Antibody Eukaryotic Expression Plasmid
[0054] 1. Construction of heavy chain and light chain variable region cDNA of monoclonal antibody
[0055] Cloning of the variable region sequence of the anti-human platelet glycoprotein GPIbα human-mouse chimeric antibody SZ-138 cell line, using 5'RACE (Rapid Amplification of cDNA Ends, rapid amplification of cDNA ends) technology, from secreted anti-human platelet The variable region sequence of the functional antibody cloned from the hybridoma cell line SZ-2A of GPIbα murine monoclonal antibody. The steps can be briefly described as follows: an antisense gene-specific primer (GSP1) is used to synthesize the first strand of cDNA, after the first strand of cDNA is purified, terminal deoxynucleotidy transferase (Terminal deoxynucleotidy transferase, TdT) is used to generate A synthetic homopolynucleotide anchor sequence is added to the 3' end of the DNA. The cDNA is amplified using a second n...
Embodiment 2
[0083] Example 2: Expression and screening of chimeric antibodies
[0084] Material:
[0085] -dhfr-deficient CHO cells were purchased from the Shanghai Cell Institute of the Chinese Academy of Sciences, and were monoclonalized and serum-free acclimated before transfection
[0086] - Calcium Transfection Kit (Invitrogen, Cat.No.K2780-01)
[0087] -MTX (Sigma Corporation, Cat.No.M9929)
[0088] 1. Cell line transfection, using the calcium phosphate method for transfection.
[0089] Cells were subcultured 24 hours before transfection, and transfection could be performed when the cell density reached 50%-60% confluence. The transfection method was strictly operated according to the method of the kit. The chimeric antibody eukaryotic expression plasmid DNA obtained in Example 1 was digested with PvuI and linearized. The cells were cultured for 24 hours under standard growth conditions, and the supernatant was taken for ELISA to detect the transient expression level; after con...
Embodiment 3
[0103] Example 3: Purification of Chimeric Antibody and Antibody Fab Fragment
[0104] 1. Purification of chimeric antibodies
[0105] Chimeric antibodies were purified by protein A affinity chromatography. The specific steps are: (1) washing the protein A affinity chromatography column with 3-5 times column volume of water. (2) Equilibrate protein A affinity chromatography column with 20mM phosphate buffer, pH 7.0. (3) Pump the cell culture supernatant containing the desired purified monoclonal antibody into the protein A affinity chromatography column. (4) Wash the column with 20mM phosphate buffer, pH 7.0, to OD 280 Figure 4 (The left side is the non-reducing gel electrophoresis of the SZ 138 IgG full-length antibody, and the right side is the reducing gel electrophoresis). The purified chimeric antibody was determined by HPLC, and its purity was 94.4%.
[0106] 2. Purification of chimeric antibody Fab fragments
[0107] Chimeric antibodies were digested with papain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com