Method for subsequent treatment of aluminum sulfate generated in technical process of extracting alumina from fly ash
A technology of process and treatment method, applied in alumina/aluminum hydroxide and other directions, can solve the problems of high investment cost, equipment corrosion, difficulty in realizing continuous production, etc., and achieve the effect of reducing costs and improving economic benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
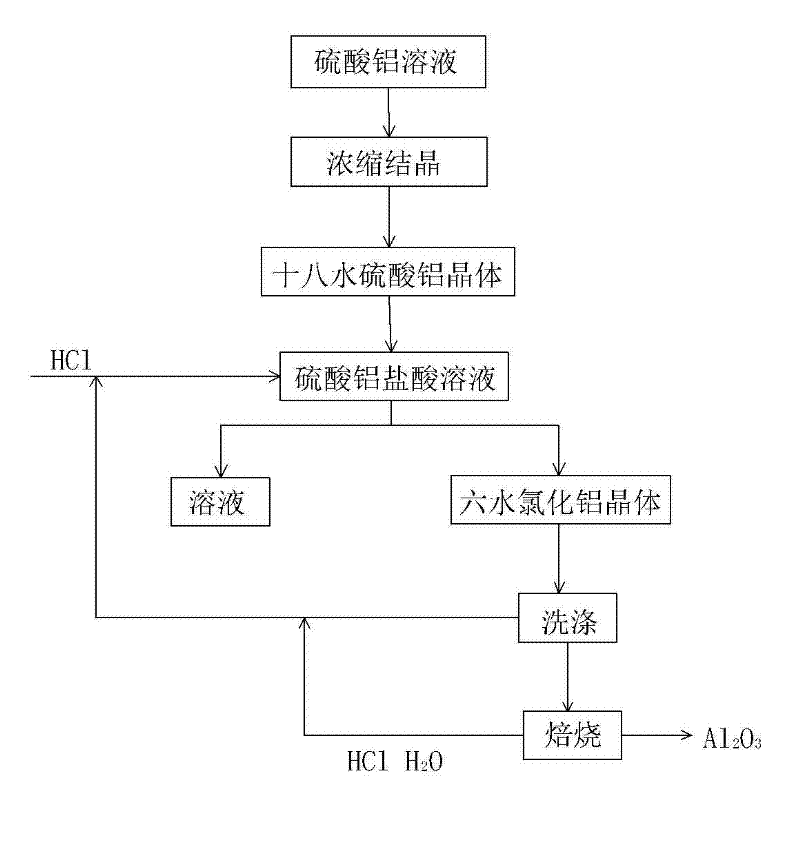
[0035] Step 1: adding aluminum chloride hexahydrate washing solution to dilute the aluminum sulfate solution obtained in the process of extracting alumina from fly ash.
[0036] Step 2: Under the temperature condition of 30° C., HCl gas is passed into the mixed solution obtained in Step 1 to make it saturated while stirring, and aluminum chloride hexahydrate crystals are precipitated;
[0037] Its reaction equation is as follows:
[0038] Al 2 (SO 4 ) 3 18H 2 O +6HCl=2(AlCl 3 ·6H 2 O)+3H 2 SO 4 +6H 2 o
[0039] Step 3: filter and wash the aluminum chloride hexahydrate crystal;
[0040] Step 4: Calcining aluminum chloride hexahydrate crystals to obtain alumina, HCl gas and water.
[0041] In step 1, the acid used in the process of extracting alumina from fly ash is sulfuric acid, and the extracted intermediate product is aluminum sulfate solution or aluminum sulfate crystals.
[0042] In step three, the aluminum chloride lotion obtained when washing the aluminum chl...
Embodiment 2
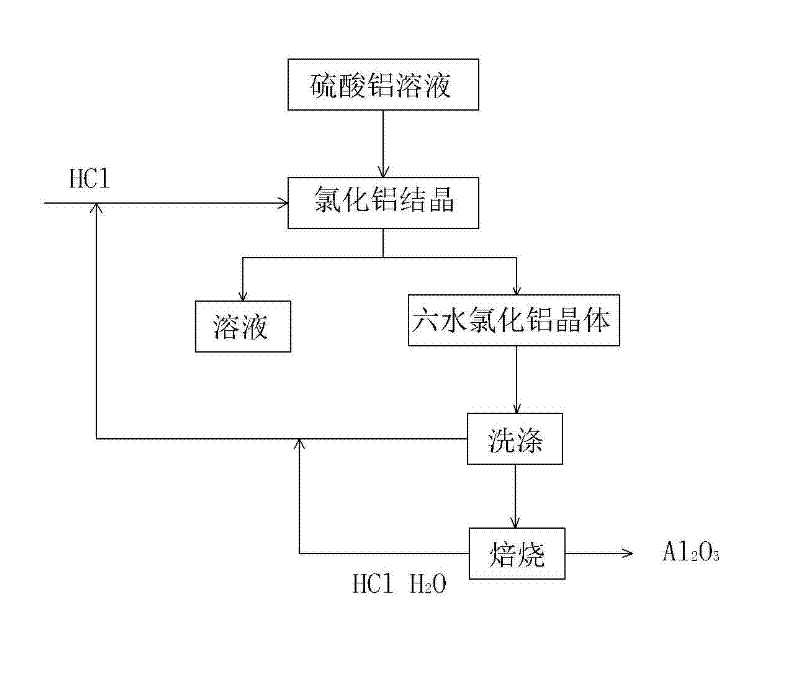
[0045] Step 1: Concentrate and crystallize aluminum sulfate crystals from the aluminum sulfate solution obtained in the process of extracting alumina from fly ash, add 20% hydrochloric acid solution for dilution, and the mass percentage of aluminum sulfate and hydrochloric acid is 1:5.
[0046] Step 2: Under the temperature condition of 30° C., HCl gas is passed into the mixed solution obtained in Step 1 to make it saturated while stirring, and aluminum chloride hexahydrate crystals are precipitated;
[0047] Its reaction equation is as follows:
[0048] Al 2 (SO4 ) 3 18H 2 O +6HCl=2(AlCl 3 ·6H 2 O)+3H 2 SO 4 +6H 2 o
[0049] Step 3: filter and wash the aluminum chloride hexahydrate crystal;
[0050] Step 4: Calcining aluminum chloride hexahydrate crystals to obtain alumina, HCl gas and water.
[0051] In step 1, the acid used in the process of extracting alumina from fly ash is sulfuric acid, and the extracted intermediate product is aluminum sulfate solution or alu...
Embodiment 3
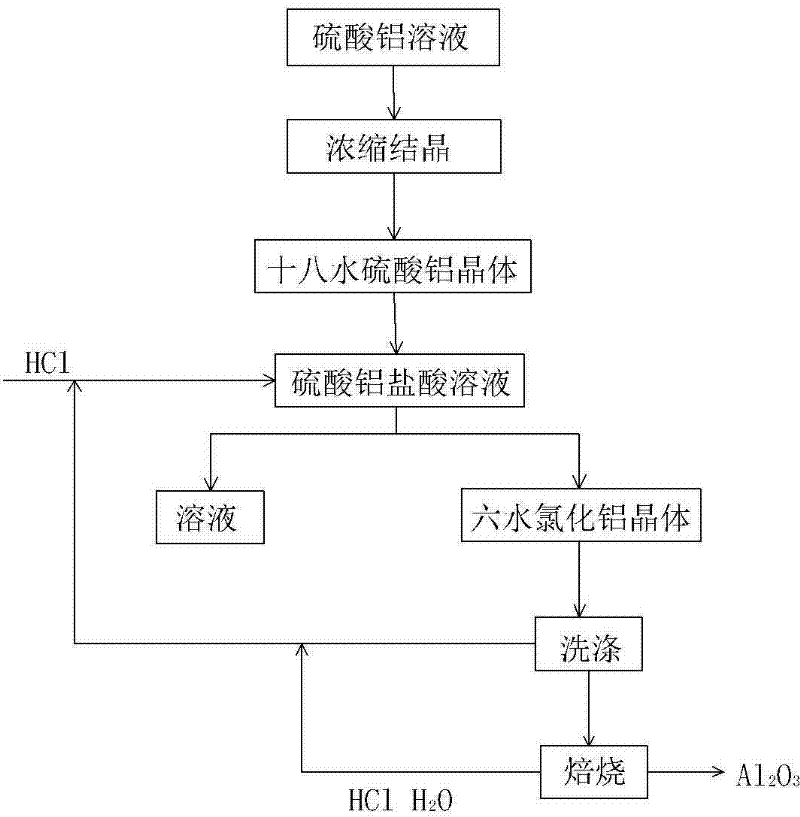
[0054] Step 1: Concentrate and crystallize aluminum sulfate crystals from the aluminum sulfate solution obtained in the process of extracting alumina from fly ash, add 28% hydrochloric acid solution for dilution, and the mass percentage of aluminum sulfate and hydrochloric acid is 1:3.
[0055] Step 2: Under the temperature condition of 55° C., HCl gas is passed into the mixed solution obtained in Step 1 to make it saturated while stirring, and aluminum chloride hexahydrate crystals are precipitated;
[0056] Its reaction equation is as follows:
[0057] Al 2 (SO 4 ) 3 18H 2 O +6HCl=2(AlCl 3 ·6H 2 O)+3H 2 SO 4 +6H 2 o
[0058] Step 3: filter and wash the aluminum chloride hexahydrate crystal;
[0059] Step 4: Calcining aluminum chloride hexahydrate crystals to obtain alumina, HCl gas and water.
[0060] In step 1, the acid used in the process of extracting alumina from fly ash is sulfuric acid, and the extracted intermediate product is aluminum sulfate solution or a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com