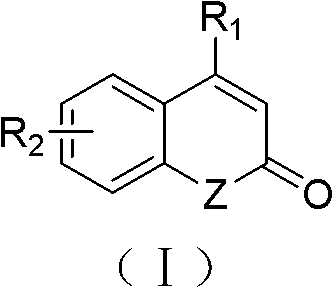
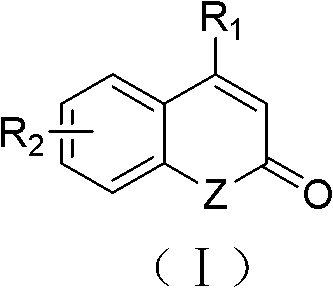
Solid-phase synthesis method of coumarin and analogue thereof
A technique of solid-phase synthesis and analogues, applied in organic chemistry and other fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
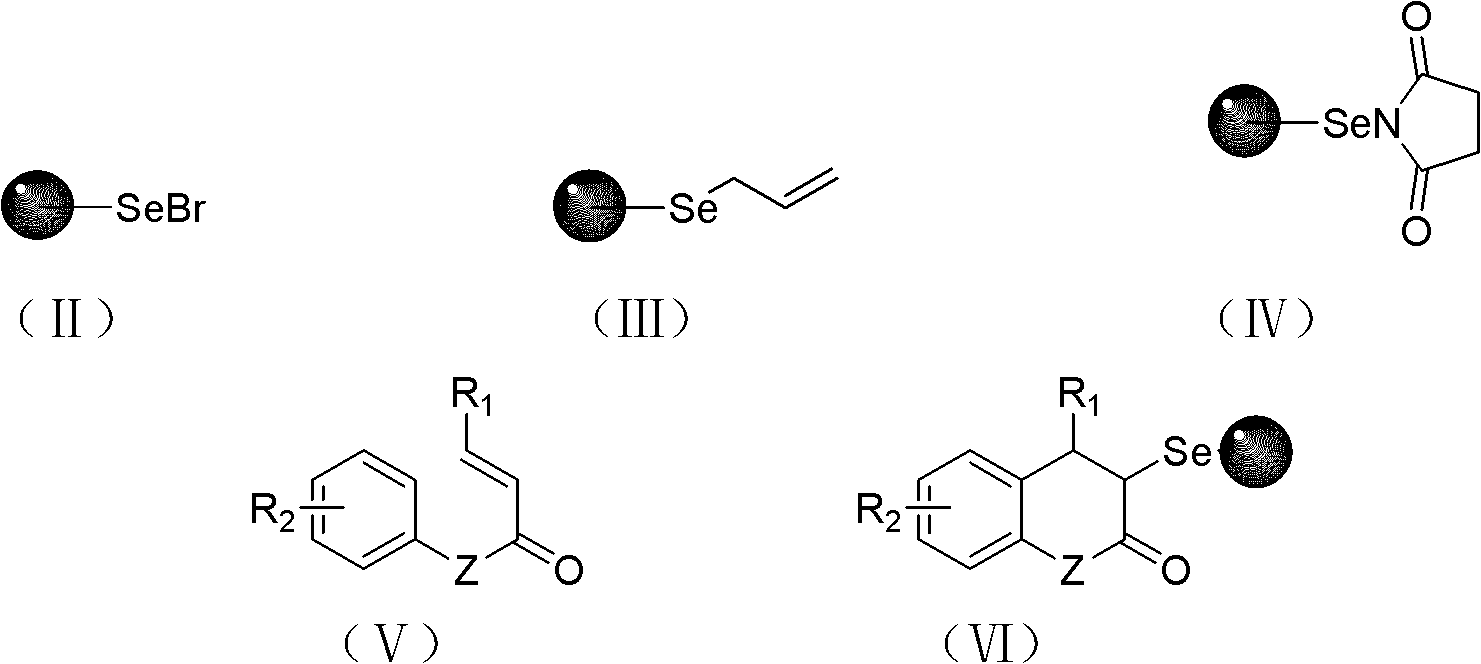
[0038] Example 2 Under nitrogen protection, 1% cross-linked polystyrene supported selenium bromide (II) (Br% = 0.99mmol / g) (2.5g, 2.48mmol), tetrahydrofuran (80mL) and N, N-di Methylformamide (20 mL) was placed in a reaction flask and allowed to stand at room temperature for 4 h. After the resin was swollen, sodium borohydride (0.473g, 12.5mmol) was added, stirred at 40°C for 2h, allyl bromide (1.572g, 13.0mmol) was added dropwise, and stirred at 40°C for 5h. After the reaction is completed, filter with a sand core funnel, and then use dichloromethane (5×10mL), water (3×10mL), water+tetrahydrofuran (V / V=1:1, 3×10mL), tetrahydrofuran (3×10mL) , ethanol (3×10mL), methanol (3×10mL), ether (3×10mL), dichloromethane (10mL), acetone (3×10mL) to wash the resin, and dry to obtain polystyrene-loaded allyl selenide (III) (2.35 g, 98.0%), IR is consistent with Example 1.
[0039] Example 3 Under nitrogen protection, 1% cross-linked polystyrene supported selenium bromide (II) (Br% = 0.9...
Embodiment 3
[0041] Example 5 Under nitrogen protection, 1% cross-linked polystyrene supported selenium bromide (II) (Br% = 0.99mmol / g) (2.5g, 2.48mmol), tetrahydrofuran (24mL) and absolute ethanol (6mL ) in a reaction flask and let it stand at room temperature for 4h. After the resin was swollen, sodium borohydride (189.2mg, 5.0mmol) was added, and the reaction was stirred at 10°C for 48h. Allyl bromide (0.665g, 5.5mmol) was added dropwise, and the reaction was continued at 20°C for 24h. After the reaction is complete, filter with a sand core funnel, and then use dichloromethane (3×10mL), water (3×10mL), water+tetrahydrofuran (V / V=1:1, 3×10mL), tetrahydrofuran (3×10mL) , ethanol (3×10mL), methanol (3×10mL), ether (3×10mL), dichloromethane (10mL), acetone (3×10mL) to wash the resin, and dry to obtain polystyrene-loaded allyl selenide (III) (2.2 g, 92.0%), IR is consistent with Example 1.
Embodiment 4
[0042] Example 6 Polystyrene-supported allyl selenide (III) (1.0 g, 0.97 mmol) and anhydrous dichloromethane (15 mL) were placed in a reaction flask, and allowed to stand at room temperature for 4 h. After the resin was swollen, N-chlorosuccinimide (0.668 g, 5.0 mmol) was added at 0° C., stirred for 5 h at 0° C., and then stirred for 24 h at room temperature. After the reaction was completed, filter with a sand core funnel, and wash the resin with dry dichloromethane (4×10 mL) to obtain polystyrene-loaded selenosuccinimide (IV). Immediately continue to soak the obtained resin (IV) with dry dichloromethane (15mL), cool to -78°C, add trimethylsilyl trifluoromethanesulfonate (0.022g, 0.10mmol), The reaction was stirred for 0.5h. 3-Phenylacrylate-4'-methylphenyl ester (V) (1.19g, 5.0mmol) was added, and the reaction mixture was kept at -20°C for 8h after continuing stirring at -78°C for 2h. After the reaction was complete, a saturated solution of sodium bicarbonate (5 mL) was ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com