Benzocyclobutene monomers containing siloxane and imide structure and synthesis and application thereof
A technology of benzocyclobutene and synthesis method, which is applied in chemical instruments and methods, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., and can solve the problems of high water absorption, high dielectric constant, polyimide The problem of amine refractory/solubility has been solved, and the effects of high production efficiency, pure product and simple preparation method have been achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Synthesis of monomer BCB-SiO-0 (I, n=0):
[0022] (a) 1.34 g (0.01 mol) of 1,1,3,3-tetramethyldisiloxane, 3.28 g (0.02 mol) of 5-norbornene-2,3-dioic anhydride and 0.032 mL of Karstedt catalyst ( Pt, ~2%) was dissolved in 10mL of toluene, stirred and reacted at 110°C for 15 hours. After the reaction solution was cooled to room temperature, 0.4 g of activated carbon was added, and stirring was continued for 0.5 hours. After filtration, toluene solvent was distilled off under reduced pressure, washed twice with anhydrous diethyl ether (2*10 mL), and vacuum-dried at 80°C for 4 hours to obtain 3.5 g of a viscous product with a yield of 75.6%.
[0023] 1 H NMR (500 MHz, CDCl 3 ) δ: 0.05-0.08 (m, 12H), 0.65 (t, 2H), 1.55-1.68 (m, 8H), 2.72-2.9 (m, 4H), 3.41-3.45(m, 4H). FTIR (KBr) : 1857 and 1775cm -1 (anhydride, ν C=O ), 1224cm -1 (ν C-Si ), 1084cm -1 (ν Si-O-Si ).
[0024] (b) Add the product obtained in step (a) (1.26g, 10.58mmol), 4-aminobenzocyclo...
Embodiment 2
[0026] Example 2 Synthesis of monomer BCB-SiO-6 (I, n≈6):
[0027] (a) 5.76 g of hydrogen-terminated polysiloxane (molecular weight ≈580 g / mol), 3.28 g (0.02 mol) of 5-norbornene-2,3-diacid anhydride, and 0.032 mL of Karstedt catalyst (Pt, ~2% ) was dissolved in 10 mL of toluene, stirred and reacted at 110°C for 14 hours. After the reaction solution was cooled to room temperature, 0.5 g of activated carbon was added, and stirring was continued for 0.5 hours. After removing the activated carbon by filtration, distilling off the toluene solvent under reduced pressure, washing with anhydrous diethyl ether (2*10 mL), and vacuum drying at 80°C for 4 hours, 7.0 g of a viscous product was obtained, with a yield of 77.1%.
[0028] 1 H NMR (500 MHz, CDCl 3) δ: 0.05-0.11 (m, 55H), 0.66 (t, 2H), 1.54-1.80 (m, 8H), 2.81-2.85 (d, 4H), 3.41-3.58(m, 4H). FTIR (KBr) : 1859 and 1782cm -1 (anhydride, ν C=O ), 1222cm -1 (ν C-Si ), 1087cm -1 (ν Si-O-Si ).
[0029] (b) Add the produc...
Embodiment 3
[0031] Example 3 Curing of monomer BCB-SiO-0 (I, n=0):
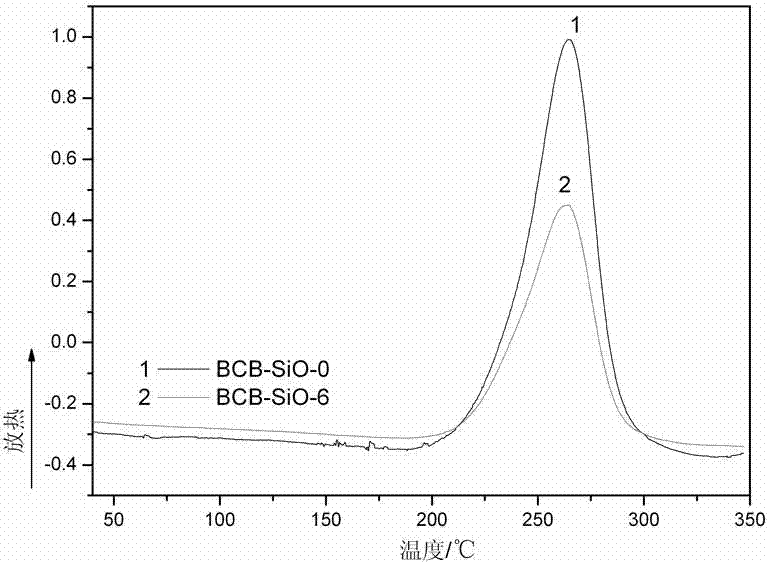
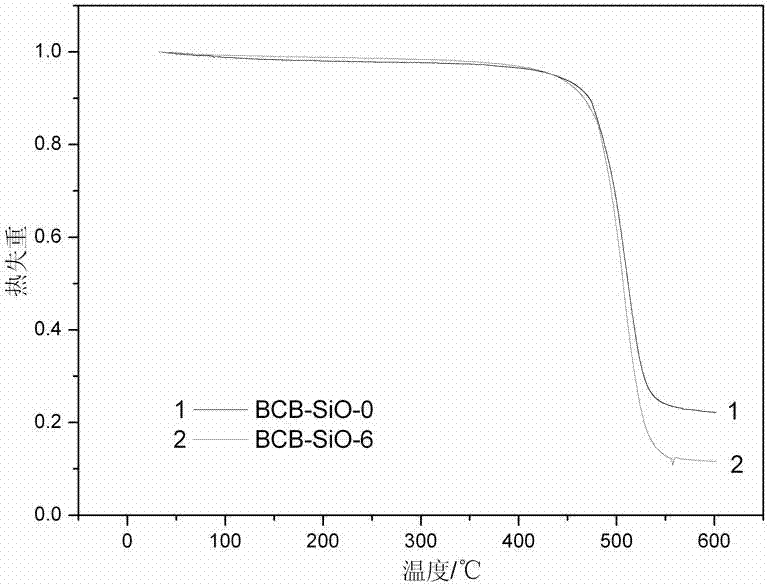
[0032] A certain amount of powdered monomer BCB-SiO-0 synthesized in Example 1 was dissolved in a small amount of CH 2 Cl 2 solvent, which is then injected into specific molds. Remove CH by volatilization at 100°C 2 Cl 2 Finally, the reaction was carried out according to the following conditions: 210°C / 1h, 250°C / 1h, 270°C / 1h, and a light yellow resin with mechanical properties was obtained. The resin structure is characterized as follows: FTIR (KBr): 1776 and 1713cm -1 (ν C=O ), 1373cm -1 (ν C-N ), 1501cm -1 (δ C-H ). Thermogravimetric analysis: The initial thermal decomposition temperature (1% weight loss) of the cured resin is 468°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com