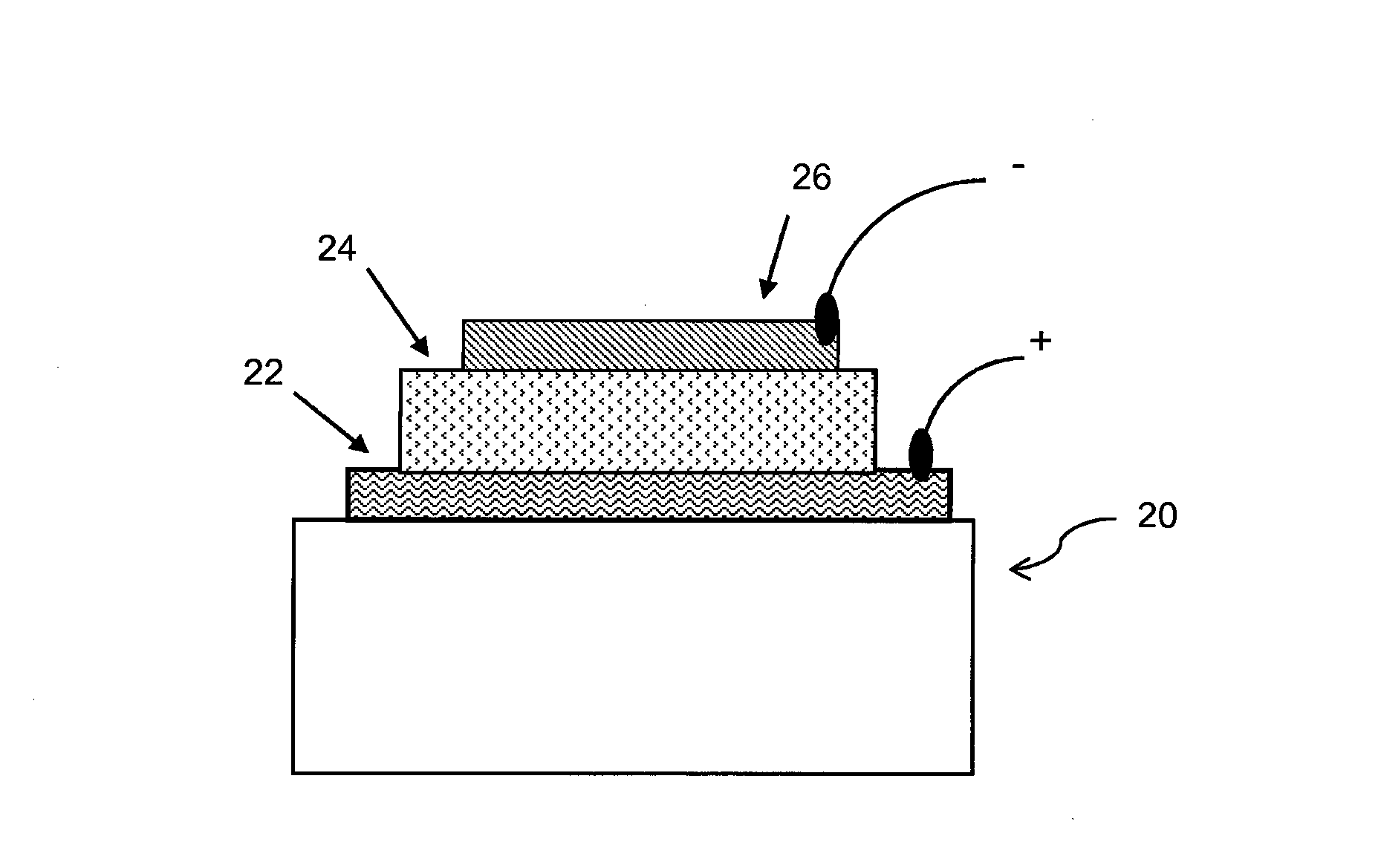
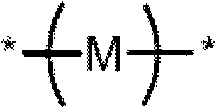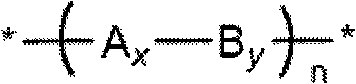Conjugated polymers and their use in optoelectronic devices
A technology of copolymerization and devices, applied in the field of conjugated polymers and their use in optoelectronic devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0186] In one aspect, the present invention relates to a method for the synthesis of oligomeric or polymeric compounds comprising repeating units of formula I, Ia or Ib in their backbone. Generally, an oligomeric or polymeric compound of the invention can be prepared having the following repeating units:
[0187]
[0188] The oligomeric or polymeric compounds are prepared in part from the following starting compounds:
[0189]
[0190] The starting compound is then functionalized to contain (a) the solubilizing group R a (eg by alkylation) and / or (b) a polymerizable group capable of self-polymerization or copolymerization with one or more other units. Such self-polymerization and copolymerization can be achieved by various polymerization systems, including metal-catalyzed coupling reactions well known to those skilled in the art, such as Stille coupling, Suzuki coupling, Negishi coupling, Kumada coupling and Yamamoto coupling. For example, the polymerizable group may b...
Embodiment 1a
[0217] Example 1a: 3,6-Bis-thieno[3,2-b]thien-2-yl-2,5-dihydro-pyrrolo Preparation of [3,4-c]pyrrole-1,4-dione
[0218] Step 1: Preparation of 3a,6a-dihydro-thieno[3,2-b]thiophene-2-carboxylic acid amide
[0219]
[0220]Heating 3a,6a-dihydro-thieno[3,2-b]thiophene-2-carboxylic acid 1 (5.0g, 26.8mmol, Fuller et al., J.Chem.Soc. (Journal of the Chemical Society), Perkin Trans 1, 3465-3470), dissolved in 50ml of thionyl chloride (SOCl 2 ) formed mixture, which was refluxed for 3 hours, and then the excess SOCl 2 Remove by rotary evaporator. Dichloromethane (125ml) was added and the resulting solution was added dropwise to NH2O in an ice / salt bath. 4 OH (150ml, 28-30%) was dissolved in a mixture formed by 200ml of dichloromethane, and the reaction temperature was kept below -5°C. A white precipitate formed immediately. The mixture was stirred for 20 minutes. The precipitate was collected by vacuum filtration and washed three times with water, and weighed after vacu...
Embodiment 1b
[0227] Example 1b: 2,5-bis-(2-butyl-octyl)-3,6-bis-thieno[3,2-b]thiophene-2- Preparation of yl-2,5-dihydro-pyrrolo[3,4-c]pyrrole-1,4-dione
[0228]
[0229] 3,6-bis-thieno[3,2-b]thiophen-2-yl-2,5-dihydro-pyrrolo[3,4-c]pyrrole-1,4-dione 4 (1.2g, 2.9mmol), 5-iodomethyl-undecane (2.59g, 8.7mmol), Cs 2 CO 3 (3.13g, 9.6mmol) was injected into a 100ml flask, then argon was bubbled in for 10 minutes, followed by the addition of 50ml of dimethylformamide (DMF). The resulting mixture was heated at 60°C for 36 hours and then cooled to room temperature. Add 50ml of chloroform. The organic layer was washed with water (50ml×3), then washed with anhydrous Na 2 SO 4 dry. The solvent was removed and a precipitate formed from methanol to give a black solid. The product was purified by column chromatography using chloroform / hexane mixture (1:1 v / v) as eluent and weighed after drying to obtain 0.78 g (yield 36%). 1 H NMR (CDCl 3 , 500MHz): δ9.28(s, 2H), 7.61(d, 2H, J=5.5), 7.30(d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| band gap | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com