Low molecular heparin iron nanoparticles and preparation method thereof
A low-molecular-weight heparin and nanoparticle technology, which is applied in the field of preparation of low-molecular-weight heparin iron nanoparticles, can solve problems such as medication inconvenience, and achieve the effect of reducing the frequency of administration and reducing the administration time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
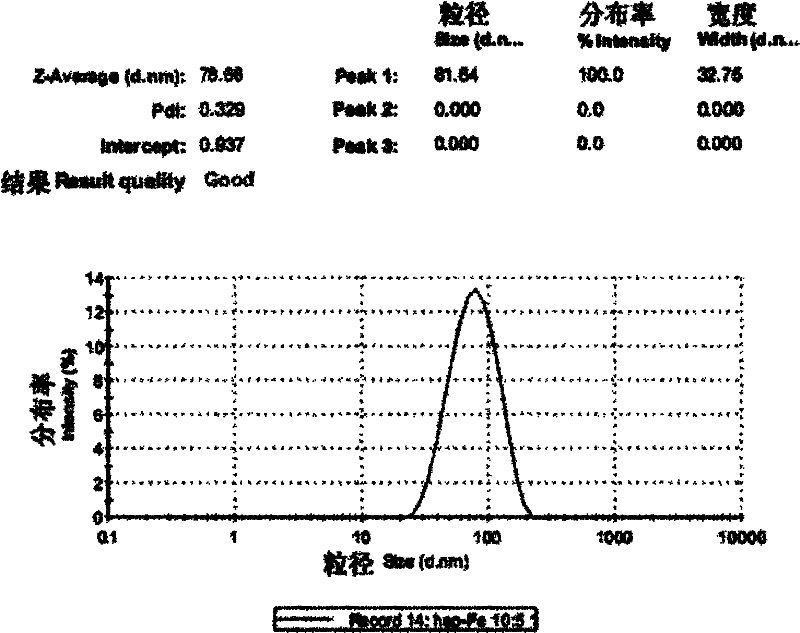
[0030] Preparation and detection of low molecular weight heparin iron nanoparticles
[0031] Method: (1) Preparation of dihydroxyl (Ⅲ) iron ion (DHOF) solution:
[0032] The mass concentration of DHOF solution is 1-20g / L, among which 5-10g / L is more suitable. FeCl with deionized water 3 ·6H 2 Dissolve O to twice the required concentration; weigh the amount of NaOH according to the molar number of Fe:OH=1:2, and dissolve it with the same volume of deionized water. Under the condition of magnetic stirring, NaOH solution was slowly dropped into FeCl 3 In the solution, a dihydroxyl (Ⅲ) iron ion solution with a pH value of 3.0 is prepared.
[0033] (2) Preparation of low molecular weight heparin (abbreviated as LMWH) solution:
[0034] The mass concentration of LMWH is 1-20g / L, among which 5-10g / L is more suitable. And add sodium chloride to the LMWH solution, the concentration of sodium chloride is 1-5g / L.
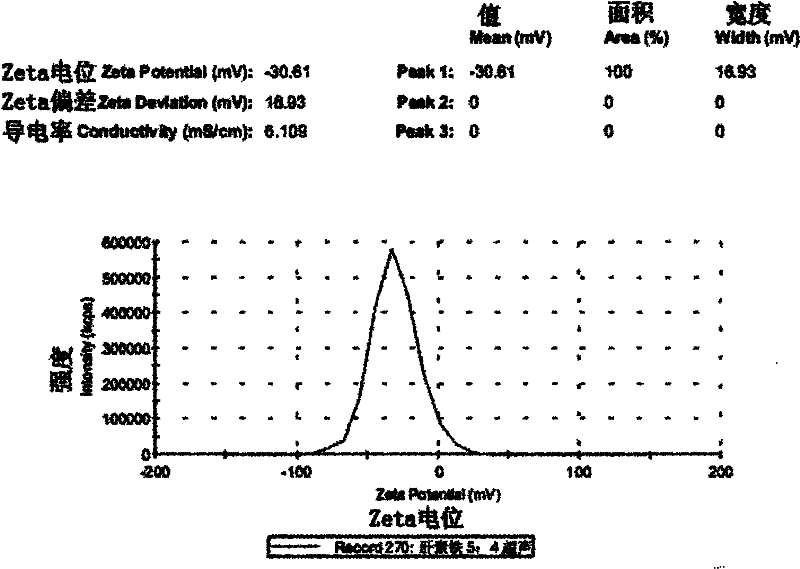
[0035] (3) Preparation of low molecular weight heparin iron nanopa...
Embodiment 2
[0046] Infrared Absorption Spectrum of Low Molecular Weight Heparin Iron Nanoparticles
[0047] Method: The mass ratio of DHOF to LMWH is 1.0, under the condition of ultrasonic vibration, the prepared low molecular weight heparin nanoparticle solution (the production method is the same as that in Example 1), and the prepared low molecular weight heparin nanoparticle solution is placed in vacuum at -54°C After drying, mix 10 mg of low-molecular-weight heparin iron nanoparticles powder and LMWH powder with 90 mg of potassium bromide powder and press them into tablets, and then use a Fourier transform infrared instrument to measure low-molecular-weight heparin iron nanoparticles and LMWH infrared. absorption spectrum.
[0048] Results: The infrared absorption spectra of low-molecular-weight heparin iron nanoparticles and LMWH are generally similar (Fourier transform infrared absorption spectra are shown in the appendix Figure 4 ). Due to [Fe(OH) 2 ] + Interacting with LMWH, ...
Embodiment 3
[0051] In vitro sustained release detection of low molecular weight heparin iron nanoparticles
[0052] Method: the prepared low-molecular-weight heparin nanoparticle solution (manufacturing method is the same as in Example 1), the nanoparticle solution was subjected to high-speed centrifugation at 10,000 rpm for 20 minutes, the supernatant was discarded, the precipitate was washed 3 times with deionized water, and 10 mg was weighed after drying. Use 10ml of PBS solution to oscillate evenly, then ultrasonically disperse evenly, put it in a dialysis bag with a molecular weight of 12000, put it into 190ml of PBS solution, shake at 80rpm, take 100ul samples from PBS on 1d, 2d, 1w, 2w, and 4w respectively, and detect its concentration of LMWH.
[0053] Results: The release of low-molecular-weight heparin nanoparticles was relatively large on the first day, accounting for about 5.1% of the total amount, followed by uniform release, and the release amount accounted for about 43.8% o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com