Isosorbide mononitrate sustained-release tablet and preparation method thereof
A technology of isosorbide dinitrate and sustained-release tablets, which is applied in the fields of medical formula, drug delivery, and cardiovascular system diseases, and can solve the problems of patients with coronary heart disease who miss the drug action time, increase drug residues, and drugs cannot take effect quickly , to achieve the effect of avoiding multi-dose resistance, delaying multi-dose resistance, and rapid onset of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
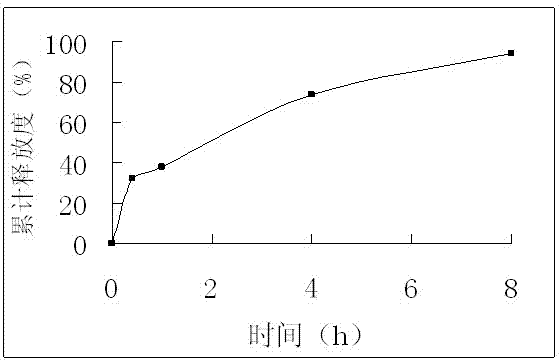
Examples
Embodiment 1
[0026] Formulation of Isosorbide Mononitrate Extended Release Tablets
[0027]
[0028] A total of 1000 tablets were pressed.
[0029] Preparation process: 1. Transfer 100g of blank microcrystalline cellulose ball cores to the fluidized bed system, add 60g of isosorbide mononitrate into the aqueous solution to dissolve, and spray 45g of isosorbide mononitrate solution into the blank microcrystalline Isosorbide mononitrate drug-loaded microspheres were prepared in a fluidized bed of cellulose spheres. In the process of preparing drug-loaded microspheres, the inlet temperature is 40°C, the outlet temperature is 35°C, and the product temperature is 35°C. In the drying process, the inlet temperature is 45°C, the output temperature is 35°C, and the product temperature is 35°C. The spray speed is 5mL / min, and the spray pressure is 2bar.
[0030] 2. Dissolve 16g of coating material in purified water. The weight ratio of hypromellose, triacetin, and talcum powder in the coating ...
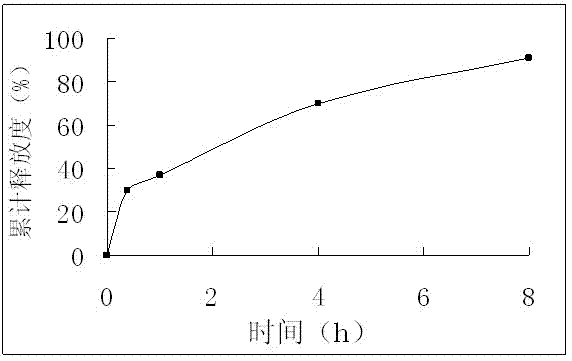
Embodiment 2
[0033] Formulation of Isosorbide Mononitrate Extended Release Tablets
[0034]
[0035] A total of 1000 tablets were pressed.
[0036] Preparation process: 1. Transfer 95g of blank microcrystalline cellulose ball cores to the fluidized bed system, add 60g of isosorbide mononitrate into the aqueous solution to dissolve, and spray 45g of isosorbide mononitrate solution into the blank microcrystalline Isosorbide mononitrate drug-loaded microspheres were prepared in a fluidized bed of cellulose spheres. Process parameter is with embodiment 1.
[0037] 2. Dissolve 12g of coating material in purified water. The weight ratio of hypromellose, triacetin, and talcum powder in the coating material is 1:0.3:0.3, and it is prepared into a coating solution. Perform coating. Process parameter is with embodiment 1.
[0038] 3. Mix the coated drug-loaded microspheres with 15 g of isosorbide mononitrate, hydroxypropyl cellulose, povidone, starch, and magnesium stearate, and then compress...
Embodiment 3
[0040] Formulation of Isosorbide Mononitrate Extended Release Tablets
[0041]
[0042] A total of 1000 tablets were pressed.
[0043] Preparation process: 1. Transfer 98g of blank microcrystalline cellulose ball cores to the fluidized bed system, add 60g of isosorbide mononitrate into the aqueous solution to dissolve, and spray 45g of isosorbide mononitrate solution into the blank microcrystalline Isosorbide mononitrate drug-loaded microspheres were prepared in a fluidized bed of cellulose spheres. Process parameter is with embodiment 1.
[0044] 2. Dissolve 17g of coating material in purified water, the weight ratio of hypromellose, triacetin, and talc in the coating material is 1:0.3:0.2, and prepare a coating solution. Perform coating. Process parameter is with embodiment 1.
[0045] 3. Mix the coated drug-loaded microspheres with 15 g of isosorbide mononitrate, hydroxypropyl cellulose, lactose, and magnesium stearate, and then compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com