Aromatization enzyme inhibitor
An aromatase and inhibitor technology, applied in the field of enzyme inhibitors, can solve the problems of lowering aldosterone level, no better curative effect than tamoxifen, poor selectivity, etc., and achieve the effect of reducing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Synthesis and aromatase inhibitory activity of methyl o-(2-ethyl-4-methylimidazol-1-methyl)phenylacetate
[0052] (1) Preparation of 2-chloromethylphenylacetic acid methyl ester
[0053] Prepared according to U.S. Patent 6048998, take isochromone (15g, 0.099mol) and dissolve it in (30.2g, 0.9mol) methanol solution, put the reaction bottle in an ice-water bath, and add chlorination dropwise at -5°C-0°C Sulfoxide (25g, 0.21mol); After the dropwise addition, be warmed up to room temperature and stirred for two hours; After the reaction, adjust the pH>6 with 10% potassium carbonate solution, extract the organic layer with methanol (75ml×3 times), and use Distill the water solution (100ml×3 times), dry over night with anhydrous magnesium sulfate, and concentrate with a rotary evaporator to obtain a colorless oily liquid, the product 2-chloromethylphenylacetic acid methyl ester 16.9g, the yield is 85%, the reaction formula is as follows :
[0054]
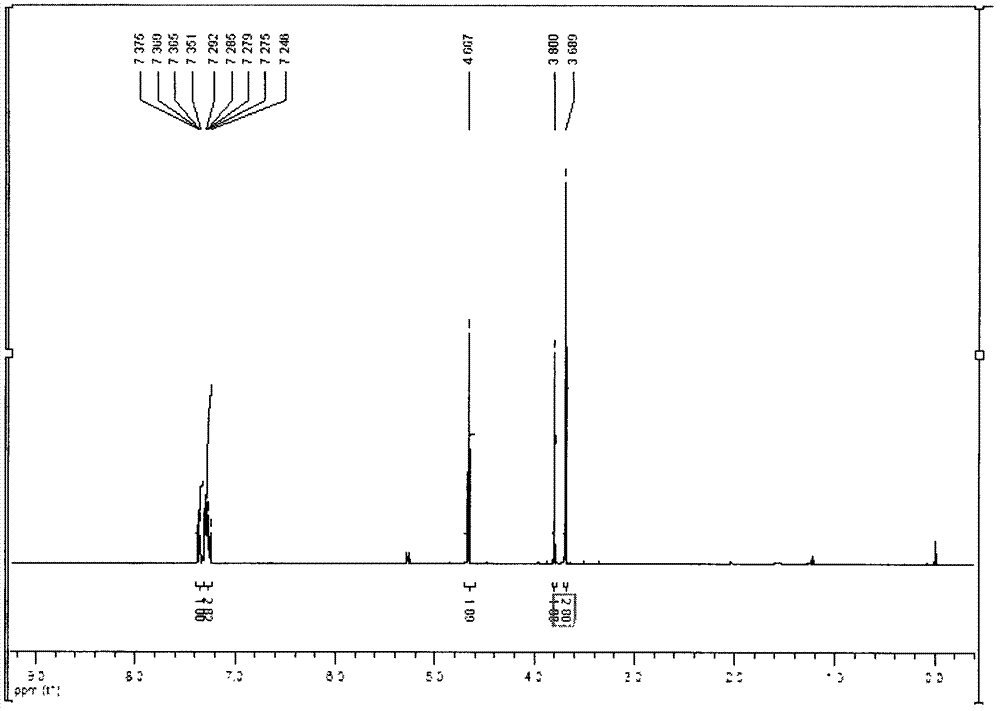
[0055] Its HNMR analy...
Embodiment 2
[0082] (1) Preparation of o-(2-ethyl-4-methylimidazolium-1-methyl)phenylacetic acid
[0083] Take the prepared o-(2-ethyl-4-methylimidazol-1-methyl)methyl phenylacetate 2.7g (10mmol) in a 100ml flask, add 10ml ethanol and 20ml 5% NaOH aqueous solution and reflux for 10h under stirring conditions Afterwards, use 6M hydrochloric acid to neutralize to partial acidity, evaporate the water to dryness under reduced pressure, extract twice with methanol after cooling, combine the methanol solution, evaporate the methanol under reduced pressure to obtain a light yellow oil, and obtain a light yellow crystal after cooling. O-(2-ethyl-4-methylimidazol-1-methyl)phenylacetic acid, the reaction formula is as follows:
[0084]
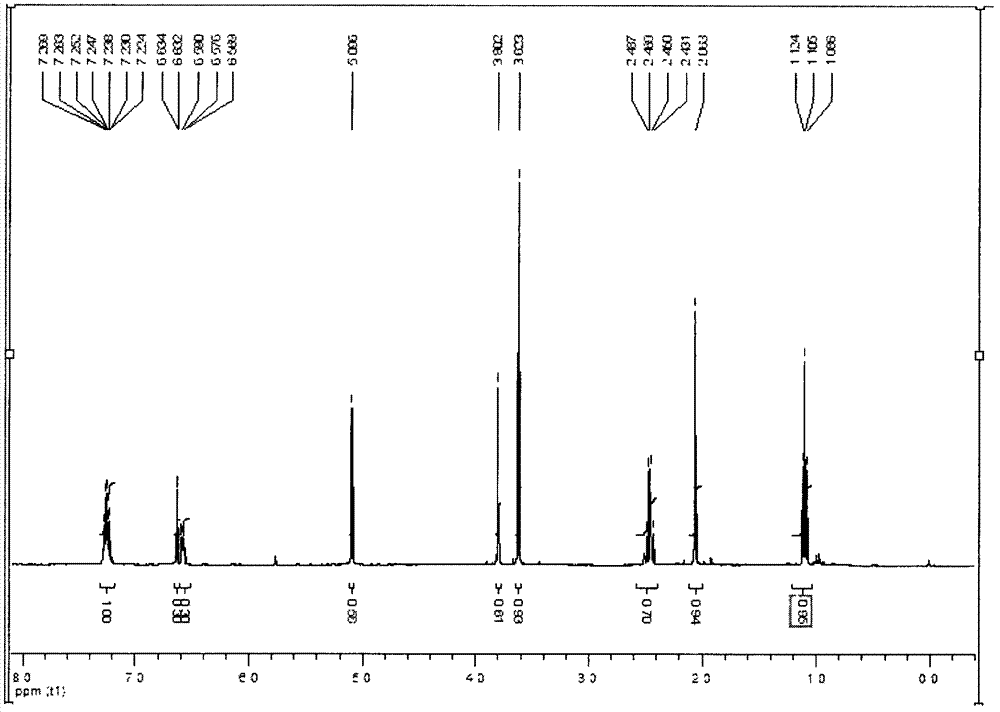
[0085] Its HNMR analysis see image 3 , where 1H NMR (CDCl 3 , ppm, 400MHz) δ1.20(t, 3H), 2.07(s, 3H), 2.53(m, 2H), 3.30(s, 3H), 3.50(s, 2H), 5.02(s, 2H), 6.60 (s, 1H), 7.22 (m, 4H) MS: m / z = 259.31 (M+H) + , elemental analysis: C 69.25; H 7.12; N 10.91.
[...
Embodiment 3
[0089] Synthesis and Aromatase Inhibitory Activity of Methyl O-(4-Formic Acid Ethylimidazol-1-Methyl) Phenylacetate
[0090] (1) Synthesis of o-(4-formic acid ethyl imidazole-1-methyl) methyl phenylacetate
[0091] Dissolve ethyl imidazole-4-carboxylate (5g, 35.68mmol) in 150ml of chloroform, add methyl 2-chloroethylphenylacetate (8.0g, 40.27mmol) under stirring, stir for 30min, then raise the temperature until reflux for 3h . Cool to room temperature, pour into distilled water, add 100ml of ethyl acetate, separate the layers and extract the organic layer (2×100ml). Dry over anhydrous magnesium sulfate overnight, and concentrate. Purification by column chromatography, eluting with ethyl acetate / petroleum ether (1:3), gave methyl o-(4-ethyl imidazole-1-methyl)phenylacetate as a white oily product with a yield of 73%. The reaction formula is as follows:
[0092]
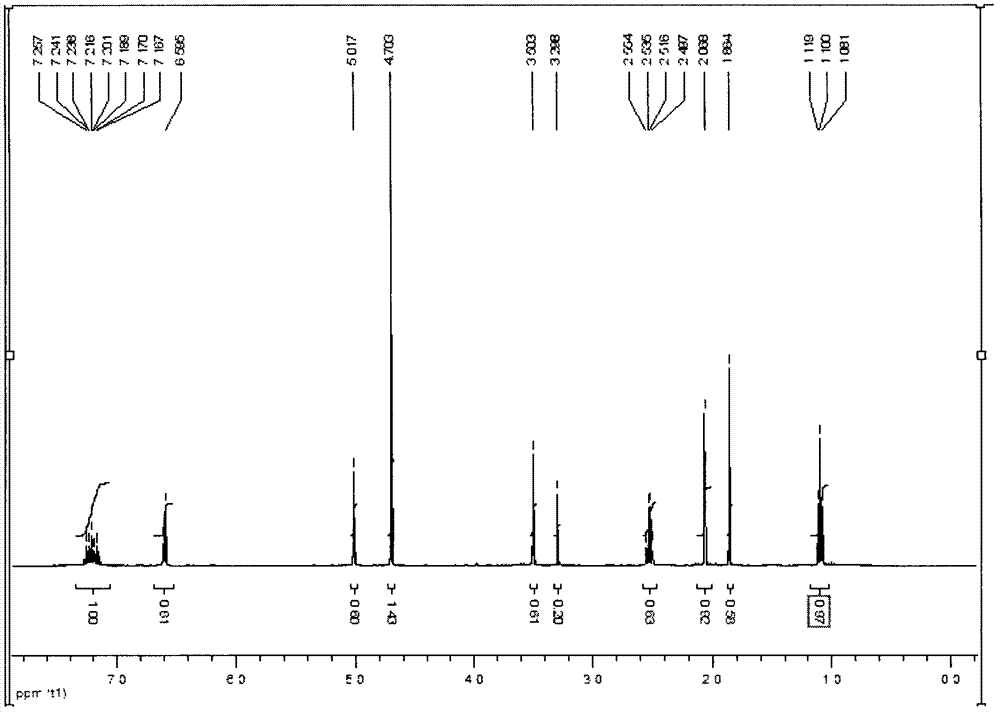
[0093] Its HNMR analysis see Figure 4 ,in,
[0094] 1H NMR (CDCl 3 , ppm, relative to TMS, 400MHz) δ1.36(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com