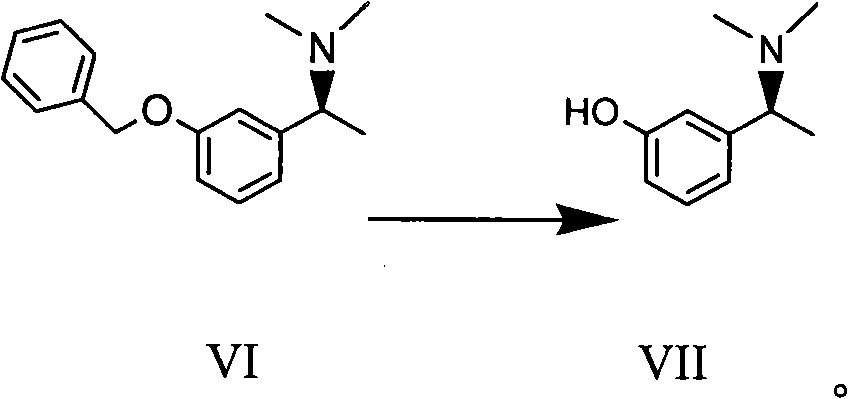
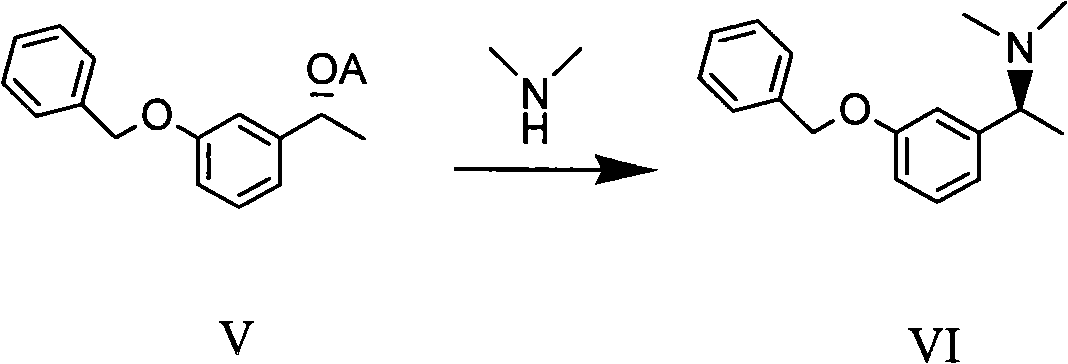
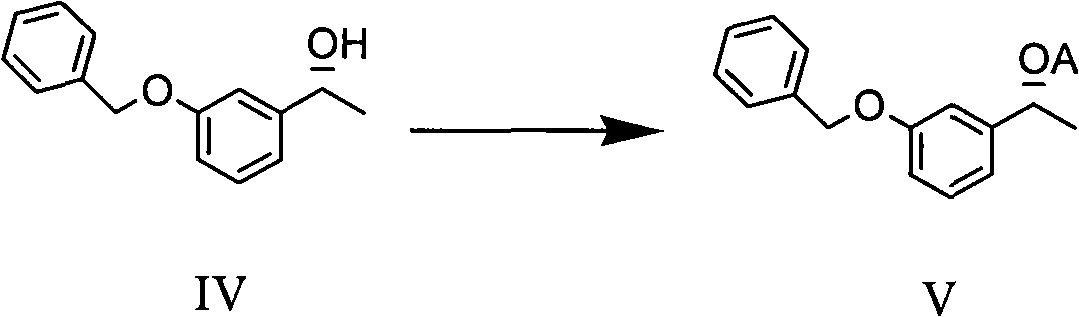
Method for preparing intermediate compound of Rivastigmine and intermediate compound
A technology for compounds and intermediates, which is applied to the preparation of intermediate compounds of rivastigmine and the field of intermediate compounds, can solve the problems of low yield, high cost, and difficulty in applying industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] The preparation of embodiment 1 m-benzyloxyacetophenone (III)
[0077] Put compound (II) 108.8g (0.8mol) into a 1L three-necked flask equipped with a thermometer and a stirrer, DMF 600ml and potassium carbonate 220.8g (1.6mol), and add benzyl chloride 126.6g (1mol) dropwise at room temperature under stirring, and then Heat to 70°C, react overnight, TLC detects that the raw materials have reacted completely, filter after cooling, evaporate the filtrate to dryness under reduced pressure, add 600ml of toluene to dissolve, then wash with saturated brine, dry, and distill under reduced pressure to obtain 164g (HPLC purity 98.4% ). Yield: 91%.
Embodiment 2
[0078] The preparation of embodiment 2 (R)-1-(3-(benzyloxy)phenyl)ethanol (IV)
[0079] Put 2.26g (10mmol) of compound (III) into the hydrogenation kettle, then add 40ml of isopropanol, 0.1ml of 1M potassium tert-butoxide in tert-butanol solution and metal catalyst RuCl under nitrogen atmosphere 2 (s) xylBinap(s)-Daipen (CAS No. 220114-01-2) (50000TON), then add hydrogen, pressure 10bar, react at room temperature for 24 hours. Conversion >99%. After separation and purification, the ee value is 99%, and the HPLC purity is 98.5%.
Embodiment 3
[0080] The preparation of embodiment 3 (R)-1-(3-(benzyloxy)phenyl)ethanol (IV)
[0081] In a 250ml autoclave, 18.08g (80mmol) of compound (III), 60g of isopropanol, 89.6mg (0.8mmol) of potassium tert-butoxide and the chiral metal catalyst RuCl 2 (s) xylBinap(s)-Daipen (TON 5000), then add hydrogen, pressure 50bar, TLC detection of raw material reaction is complete, after vacuum evaporation to dryness 18.4g (HPLC purity 98.5%, ee value 99.9%). Yield: 99.3%.
[0082] 1 H NMR (CDCl 3 , 400M) δ: 1.4(d, 3H); 1.7(d, 1H); 4.8(m, 1H); 5.0(s, 2H); 6.8-7.4(m, 9H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com