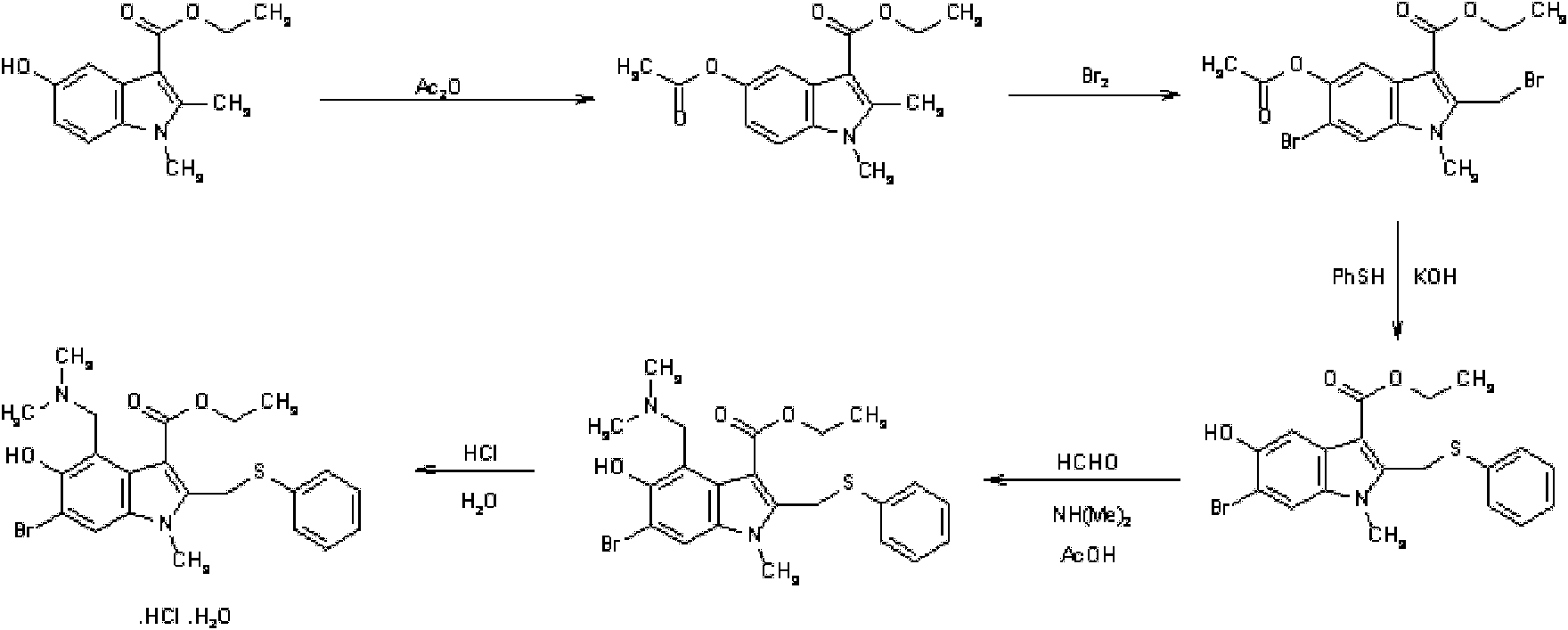
Preparation method of arbidol hydrochloride
A technology of arbidol hydrochloride and compound, applied in the field of preparation of arbidol hydrochloride, can solve the problems of low reaction yield, low total yield and the like, and achieve high yield, low manufacturing cost and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] A kind of preparation method of Arbidol hydrochloride, its steps are (preparation compound 1):
[0062] A. Preparation of compound 1: 53g of 3-iodo-4-nitrophenol was added to 160g of acetone (dried through anhydrous potassium carbonate), 30.3g of triethylamine was added, and 37.7g of triethylamine was added dropwise at room temperature (20-25°C, the same below). g of acetyl chloride, drop it within 1 hour, the reaction liquid will automatically rise to the reflux temperature T=56°C, react for 0.5h, and naturally cool to room temperature T=25°C, pour the reaction liquid into 1000g of ice water, stir, filter, and wash the filter cake with water , and dried in vacuo to obtain 57.4 g of crude compound 1 with a yield of 93.6%. The next reaction was carried out directly without further purification.
[0063] B. Preparation of compound 2: Add 48.6 g of ethyl acetoacetate and 180 ml of freshly distilled tetrahydrofuran into a dry flask. Within 2 hours, 41.9 g of potassium ter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com