Method for detecting content of Danshensu in Fuyankang tablets
A technology of Fuyankang Tablets and determination methods, applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve the effects of easy popularization, good repeatability, and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
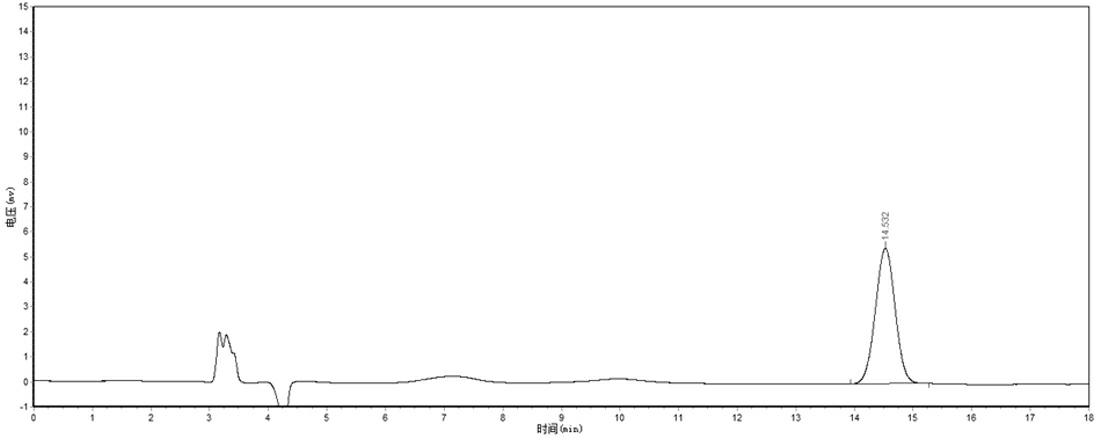
[0023] 1) Take 10 Fuyankang Tablets, remove the sugar coating from the sugar-coated tablets, grind finely, take about 1.0g, weigh it accurately, put it in a stoppered Erlenmeyer flask, and add methanol-0.5% glacial acetic acid solution (methanol and 0.5% Glacial acetic acid solution (volume ratio 1:1) 20ml, plugged tightly, weighed, ultrasonically treated (power 150W, frequency 40kHz) for 20 minutes, allowed to cool at room temperature, weighed again, and methanol-0.5% glacial acetic acid solution (including methanol and 0.5% glacial acetic acid solution (volume ratio 1:1) to make up for the lost weight, shake well, filter, and take the subsequent filtrate to obtain the sample solution of Fuyankang Tablets to be tested;
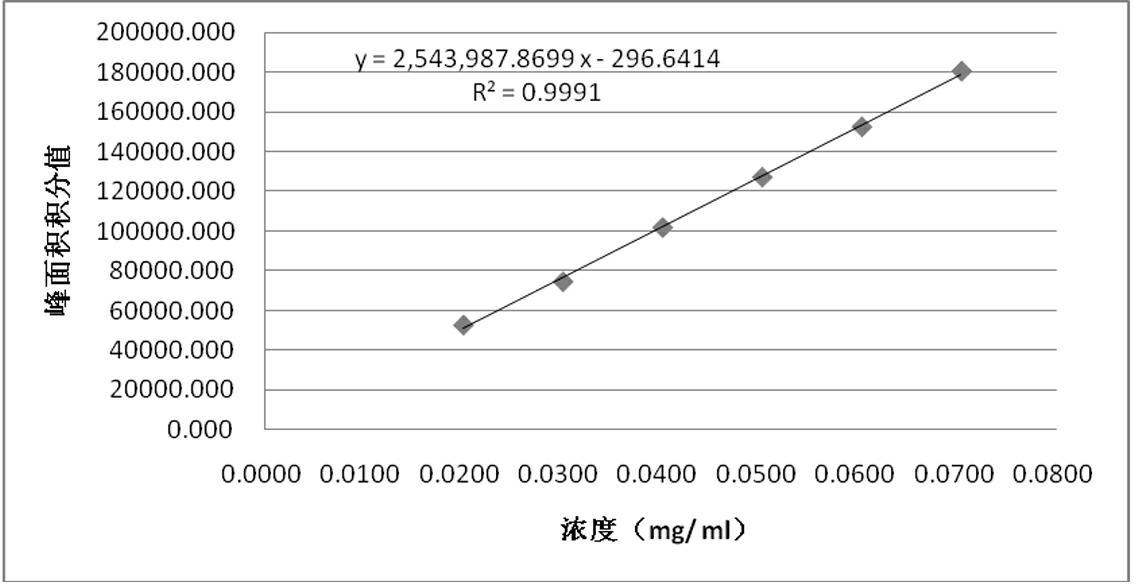
[0024] 2) Accurately weigh 12.58 mg of Danshensu sodium reference substance which has been dried under reduced pressure by phosphorus pentoxide for 12 hours, put it in a 25 ml measuring bottle, dissolve it with 70% methanol and dilute to the mark, and shake we...
Embodiment 2
[0028] Embodiment 2 repeatability test
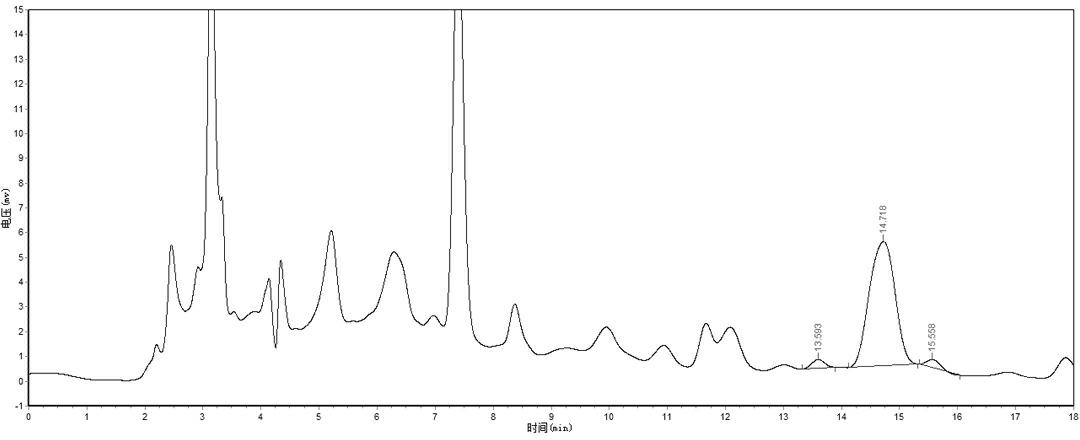
[0029] Take the same batch number (batch number: 20080724) 6 samples of Fuyankang Tablet sample fine powder, follow the step (1) of Example 1 to prepare Fuyankang Tablet to be tested sample solution and operate in parallel, and accurately absorb the reference substance Solution (0.0503 mg / ml) and 10 μl of the sample solution of Fuyankang Tablet to be tested were injected into the liquid chromatograph for determination. The results are shown in Table 2. The results show that the method has good repeatability.
[0030]
Embodiment 3
[0031] Embodiment 3 accuracy test
[0032] Take the same batch number (080724) Fuyankang tablet sample powder (containing Danshensu 0.19mg / tablet) 6 parts, about 1.25g, accurately weighed, put into a 50ml measuring bottle, and accurately add the reference substance solution (0.0503 mg / ml) 25ml, operate in parallel according to the method under step (1) of Example 1, accurately draw 10 μl, inject it into a liquid chromatograph, and measure it. The results are shown in Table 3, and the results show that the recovery rate of this method is good.
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com