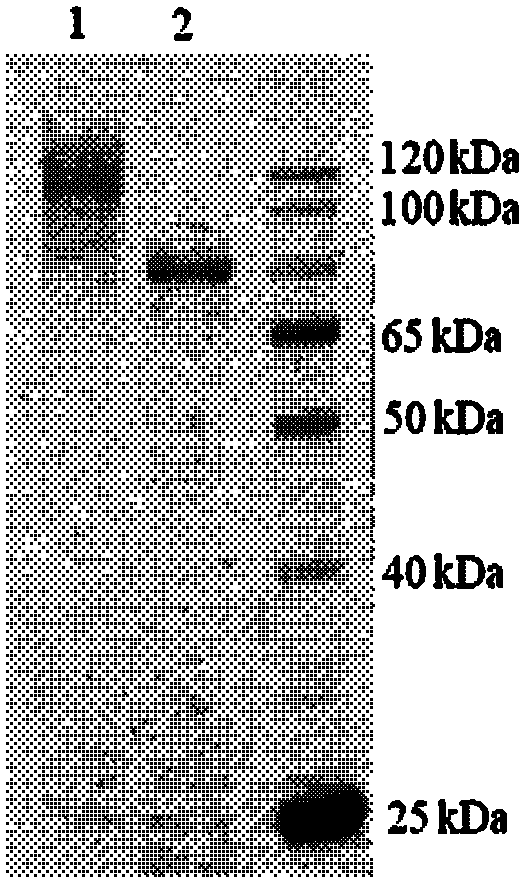
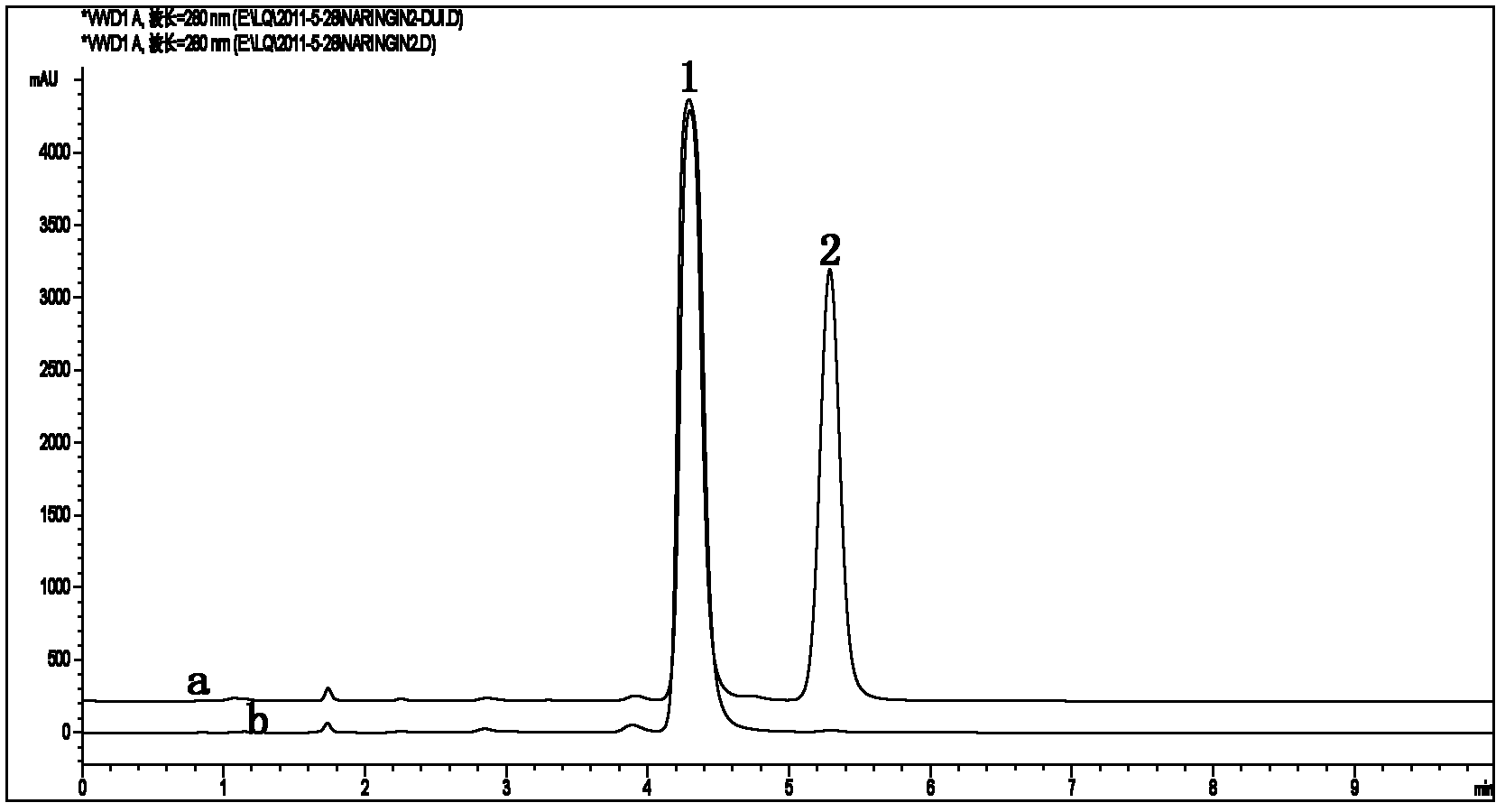
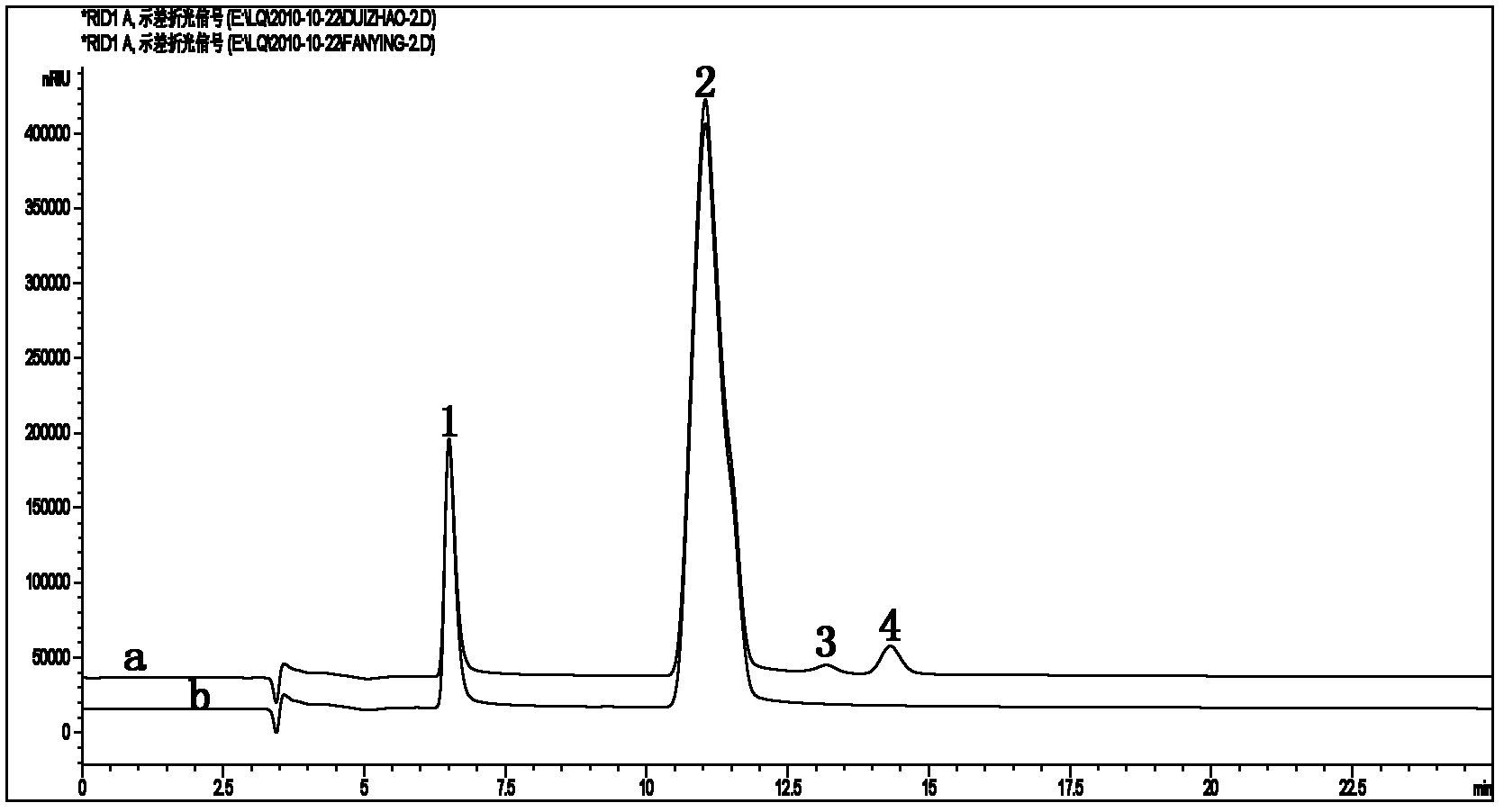
Alternaria alpha-L-rhamnosidase gene and application thereof
A technology of rhamnosidase and gene, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Extraction of Alternaria sp.L1 total RNA
[0029] Inoculate Alternaria sp.L1, strain preservation number CGMCC No.3688, into 150ml of enzyme-producing fermentation broth, culture at 28°C for 32h, and wash the cells three times with DEPC-treated ultrapure water , filter paper to collect the bacteria, and liquid nitrogen to grind and break the wall. Take 100mg of bacterial cells, add 1ml Takara RNAiso Plus, mix well, let stand at 15-30°C for 5min, then add 200μL chloroform, shake for 15s, let stand at room temperature for 5min, centrifuge at 12000 rpm, 4°C for 30min, and remove the supernatant Transfer to a new tube, add an equal volume of isopropanol, mix upside down, let stand at 15-30°C for 10min, centrifuge at 2000 rpm, 4°C for 10min, remove the supernatant; add 1mL of 75% ethanol to wash the precipitate, 7500 Centrifuge at 4°C for 5 minutes to remove ethanol, and dry for 5 minutes; dissolve the precipitate in a certain amount of DEPC-treated sterilized ul...
Embodiment 2
[0031] Embodiment 2: PCR amplification of Alternaria L1α-L-rhamnosidase gene partial cDNA sequence
[0032] The cDNA sequences of α-L-rhamnosidase genes of different strains were retrieved from GenBank, and a pair of degenerate PCR primers (N-TER and G1-R) were designed:
[0033] oligo-dT: 5′-TTTTTTTTTTTTTTTTTT-3′SEQ ID NO.6
[0034] N-TER: 5′-ACCCCHTACTCNCARTACATCYTCGC-3′SEQ ID NO.7
[0035] G1-R: 5′-GGRCCNGYRGACCANCCRTG-3′SEQ ID NO.8
[0036] Using the extracted Alternaria total RNA as a template, use the above-mentioned oligo-dT primers to carry out reverse transcription to synthesize the first strand of cDNA. The total reaction volume is 20 μL. A total of 12 μL of ultrapure water for the enzyme was pre-denatured at 65°C for 5 minutes, and placed on ice; then, 4 μL of 5× cDNA synthesis buffer, 1 μL of RNase inhibitor (20 U / μL), 2 μL of 10 mM dNTP, and reverse transcriptase ( 200U / μL) 1 μL, mix well, extend at 42°C for 60 minutes, and terminate the reaction at 70°C for 5 ...
Embodiment 3
[0037] Embodiment 3: Amplification of Alternaria L1α-L-rhamnosidase gene cDNA 3' end sequence
[0038] According to the sequence of SEQ ID No.3, in addition to N-TER, a nested PCR sense primer (F2) was designed, and the 3' end fragment of α-L-rhamnosidase gene cDNA was synthesized by 3'RACE method. The primer sequences are as follows:
[0039] 3'AP: 5'-GGCCACGCGTCGACTAGTACTTTTTTTTTTTTTTTTT-3'SEQ ID NO.9
[0040] First-round PCR primers
[0041] N-TER: 5′-ACCCCHTACTCNCARTACATCYTCGC-3′SEQ ID NO.10
[0042] 3'AUAP: 5'-GGCCACGCGTCGACTAGTAC-3'SEQ ID NO.11
[0043] Second round of PCR primers
[0044] F2: 5'-CTGGCTTCGCTTCGGTATGGG-3' SEQ ID NO.12
[0045] 3'AUAP: 5'-GGCCACGCGTCGACTAGTAC-3'SEQ ID NO.13
[0046] Using the extracted Alternaria total RNA as a template, the first strand of cDNA was synthesized by reverse transcription using the above-mentioned 3'AP primer. Using the first strand of cDNA as a template, N-TER and 3'AUAP primers were used for the first round of PCR ampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com