Low-concentration viscoelastic surfactant solution and preparation method thereof
A technology of surfactant and viscoelasticity, which is applied in the field of low-concentration viscoelastic surfactant solution and its preparation, which can solve the problems of difficult post-processing and high concentration of surfactant, achieve good viscoelasticity and reduce the cost of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
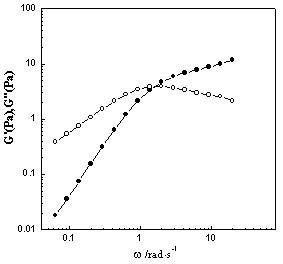
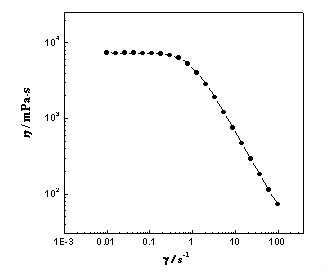
[0022] A method for preparing a low-concentration viscoelastic surfactant solution, first preparing a Gemini surfactant, then treating it with a strong basic ion exchange resin to obtain the corresponding quaternary ammonium base, and then obtaining the surfactant through acid-base neutralization The active ingredient is finally compounded with an inorganic salt to obtain the viscoelastic surfactant; wherein, the inorganic salt is sodium nitrate, and the Gemini surfactant is an oleic acid-based Gemini surfactant 18:1-2 -18:1 is obtained by the following reaction:
[0023]
[0024] Among them, oleyl alcohol can be directly purchased from the market, or can be prepared from natural product oleic acid through esterification and reduction. The preparation reaction formula is as follows:
[0025]
[0026] In the alcohol bromination step, oleyl alcohol is first reacted with p-toluenesulfonyl chloride, and then reacted with anhydrous lithium bromide. The molar ratio of oleyl a...
Embodiment 1
[0043] (1) Synthesis of methyl oleate
[0044] Add oleic acid (120 g), anhydrous methanol (120 g) and concentrated sulfuric acid (1 g) into a 500 mL single-necked bottle, and stir and reflux at 82° C. for 6 hours. After cooling, the low boiling point substances were removed, and the organic layer was washed with dilute sodium bicarbonate solution until neutral. Add anhydrous magnesium sulfate and dry overnight. After filtration, the crude product was distilled under reduced pressure to obtain methyl oleate. 176~178℃ / 6mmHg(uncorrected) Yield: 90.0%
[0045] 1 H NMR (300MHz, CDCl 3 , Me 4 Si) δ (ppm): 5.35 (m, 2H, CH=CH), 3.66 (s, 3H, OCH 3 ), 2.30(t, 2H, CH 2 COOCH 3 ), 2.02(t, 4H, CH 2 CH=CHCH 2 ), 1.62(t, 2H, CH 2 CH 2 COOCH 3 ), 1.30~1.27(m, 20H, CH 3 (CH 2 ) 6 CH 2 CH=CHCH 2 (CH 2 ) 4 ), 0.88(t, 3H, CH 3 )
[0046] (2) Synthesis of oleyl alcohol
[0047]Add methyl oleate (52 g) and tetrahydrofuran (207 g) after sodium-benzophenone reflux to remove wat...
Embodiment 2
[0066] Synthesis of 1-bromo-cis-9-octadecene:
[0067] Using commercially available oleyl alcohol, take 50g oleyl alcohol and 36g p-toluenesulfonyl chloride, the molar ratio of the two is 1:1, and react at -20°C for 15 hours in the presence of 10 times the molar amount of triethylamine. After washing with water and drying, the p-toluenesulfonate of oleyl alcohol is obtained. 80 g of anhydrous lithium bromide was added in a 5-fold molar amount, and acetone was added at the same time, and the reaction was carried out at room temperature for 2 hours. Acetone was evaporated to dryness, petroleum ether was added, and after standing at -5°C, 1-bromo-cis-9-octadecene was obtained by filtration, solvent removal, and column chromatography with a yield of 21.5%.
[0068] Quaternization:
[0069] 1-Bromo-cis-9-octadecene (40g, 0.12mol), N,N,N',N'-tetramethylethylenediamine (5g, 0.04mol), the molar ratio of the two is 3: 1. Add 150mL of anhydrous acetonitrile into a 500mL single-necked...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Zero shear viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com