New compound separated from Mythic Fungus, preparation method thereof and medicinal purpose thereof
A compound and drug technology, applied in the field of new compounds for multi-drug resistance (MDR), can solve problems that have not been widely used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 compound (1) preparation
[0030] The material source Ganodema Lucidum (Leys.ex Fr.) KARST) is purchased from China Fujian Xianzhi Tower Biotechnology Co., Ltd., which is stored at the School of Pharmacy of Fujian Medical University.
[0031]Extract and separate dry and crushed ganoderma with water -free ethanol to extract 3 times, 2h each time, filter with No. 2 filter paper, and remove ethanol with a rotating evaporator to get alcohol extraction.After adding an appropriate amount of water to the alcohol, use petroleum ether and ethyl acetate to extract in turn.Perform the column layer on the silicone, elute it with chloroform-methanol gradient, and take chloroform-methanol (volume ratio 99: 1) to cricket column layer.Ether-ethyl acetate (volume ratio ratio 4: 1) to elute, take this part of the preparation of high-efficiency liquid chromatography (Shimadzu LC-6AD semi-preparation high-efficiency liquid chromatography instrument), methanol-water gradient to eluted...
Embodiment 2
[0032] Example 2 compounds (1) chemical structure determination
[0033] Structural measurement is used to record the FTir spectrum with Perkin-Elmer 1600 optical light meter, and the ultraviolet spectrum is measured with Shimadzu-3100 Lighting Turn. 3 In the solution, the Bruker MRI can record the NMR spectrum, and use the Agilent 6210 flight timermia.
[0034] The physical and chemical properties of compounds (1) are the invention compounds (1) are white and foster powder, UV (ETOH) λmax 254nm; Liebermann-Burchard reaction positive; IR νmax (CM -1 ): 3427, 2971, 2917, 2888, 1725, 1703, 1652, 1453, 1408, 1380, 1306, 1239, 1056; 1 H-nmr (CDCL 3 , 500MHz): Δ1.52 (1H, M, H-1), Δ2.90 (1H, M, H-1 '), Δ2.50 (1H, M, H-2), Δ2.45M, H-2 '), Δ1.55 (1H, M, H-5), Δ2.11 (1H, M, H-6), Δ1.64 (1H, M, H-6'), Δ4.81 (1H, M, H-7), Δ2.79 (1H, D, J = 8.5Hz, H-12), Δ2.75 (1H, D, J = 8.5Hz, H-12 '), Δ2.12 (1H, DD, J = 19.5, 9.5 Hz, H-16), Δ2.79 (1H, DD, J = 19.5, 8.5Hz, H-16 '), Δ1.97 (1H, M, H-17), Δ0.9...
Embodiment 3
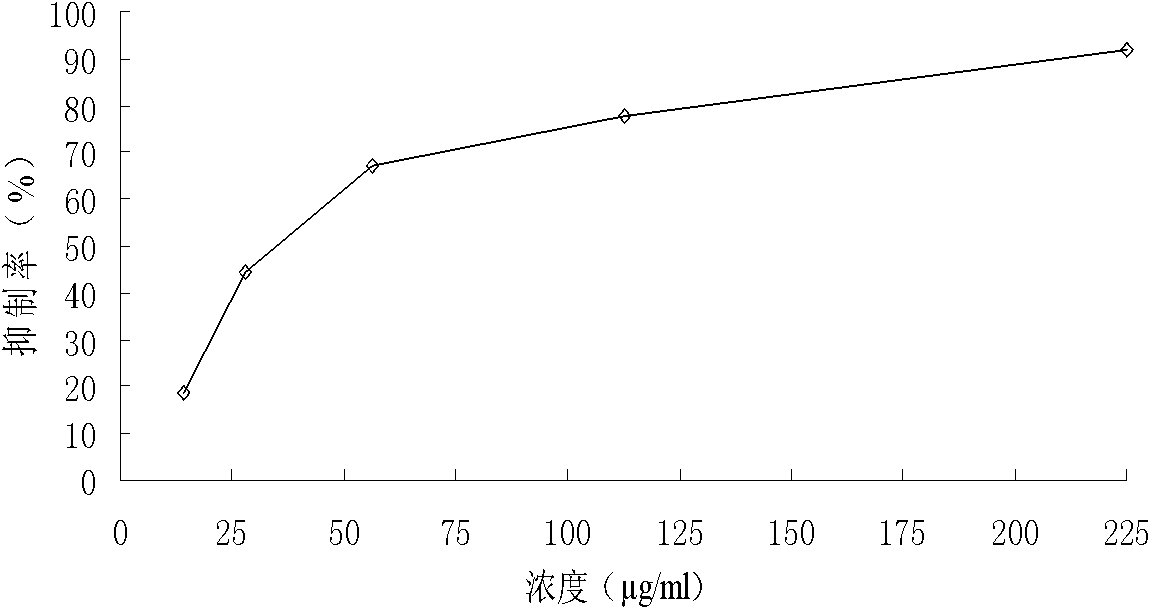
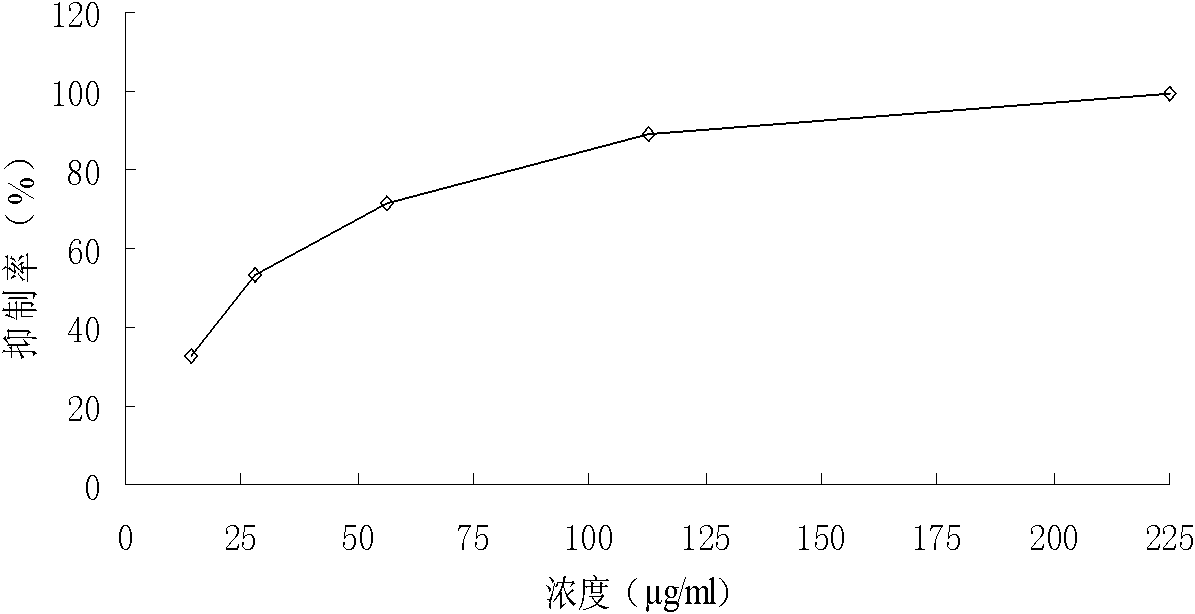
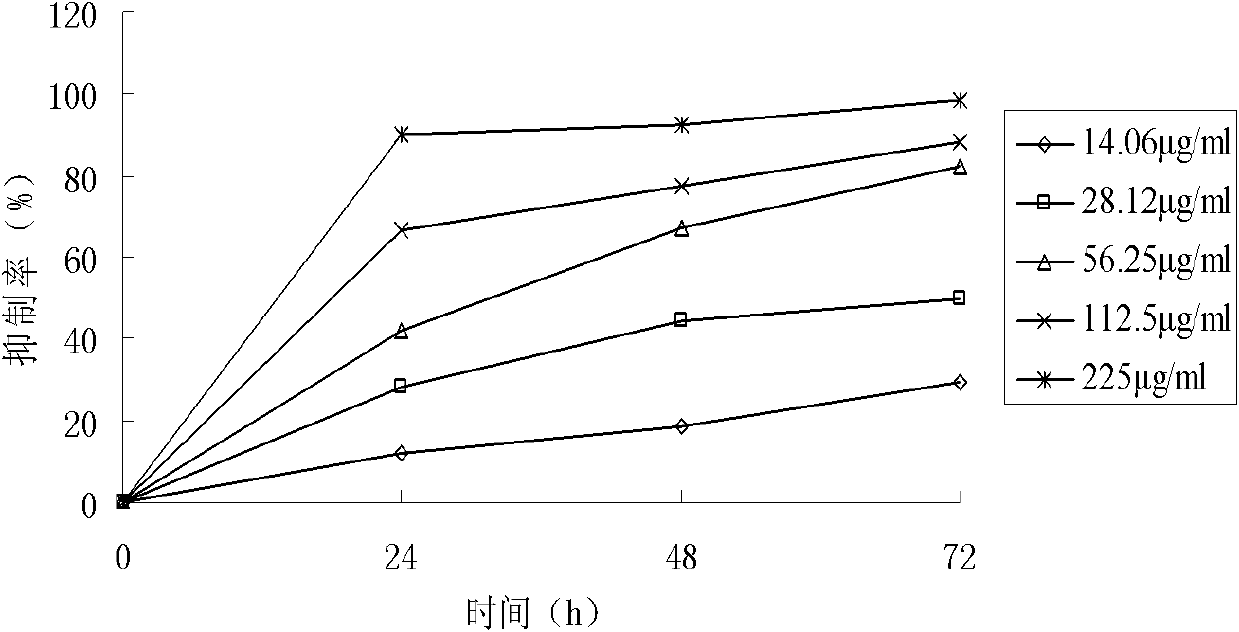
[0037] Example 3 compounds (1) Biology experiments and analysis of anti -cancer effects
[0038] 1. Materials and methods
[0039] Cell and reagent high-expressed drug-resistant cells K562 / A02, human chronic granulocyte leukemia cells K562, human marrow leukemia cell plant HL60, human Burkitt lymphoma cell CA46, human colon cancer cell strain SW1116Human colon cancer cells SW480, human liver cancer cells SMMC7221, and human liver cancer cells HEPG2.Cultivate these cells in the RPMI 1640 culture liquid containing 10%calf serum, set 37 ° C, 5%saturated humidity CO 2 Cultivation in the culture box (SMMC7221 of human liver cancer cells and human colon cancer cells SW480 are cultivated with 10%Maverick serum culture liquid).The above compounds (1) chemical names are Achide Athyl, which are obtained from the preparation of Example 1.
[0040] Cell proliferation analysis takes the number of K562, HL60, CA46, SW1116, SW480, SMMC7221 and HEPG2 cells, and inoculate in a certain density in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com