Epitope of Epstein-Barr virus latent membrane protein 2b and its application
A latent membrane protein and Epstein-Barr virus technology, applied in antiviral immunoglobulin, application, antiviral agents, etc., can solve the problem of weak immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0096] The present invention also provides a kit (kit) for detecting diseases related to Epstein-Barr virus infection. The kit contains: a solid phase carrier coated with the B cell surface bit recombinant protein. The preparation method of the kit (kit) includes: (1) coating the B cell epitope recombinant protein on a solid phase carrier (such as an ELISA reaction plate), and obtaining the B cell epitope coated and (2) placing the solid phase carrier coated with the B cell epitope recombinant protein obtained in (1) into a kit, thereby obtaining a drug for detecting Epstein-Barr virus infection-related diseases box. The kit may also include reagents (such as enzyme-linked immunosorbent reagents) for detecting antigen-antibody reactions, or reagents for gene amplification (such as PCR reagents) in appropriate containers, and / or also Instructions for use (book) are included.
[0097] Detection purpose
[0098] The B cell epitope recombinant protein of the invention can be u...
Embodiment 1
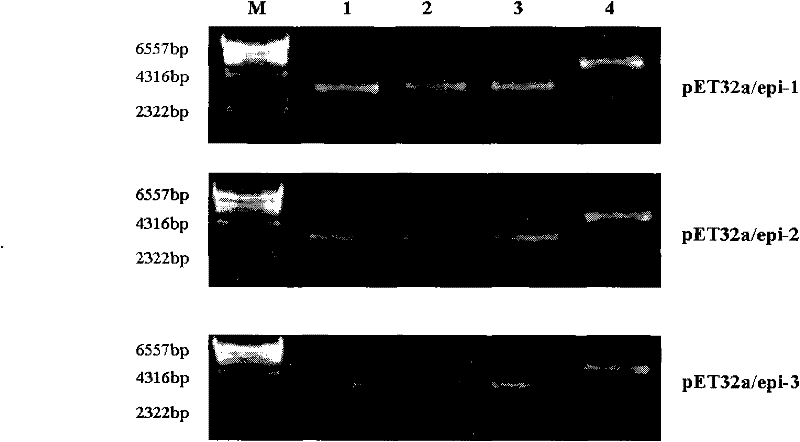
[0108] Example 1, Preparation and Identification of EBV-LMP2B Cell Epitope Recombinant Protein
[0109] 1. Design of EBV-LMP2B cell epitope recombinant protein gene
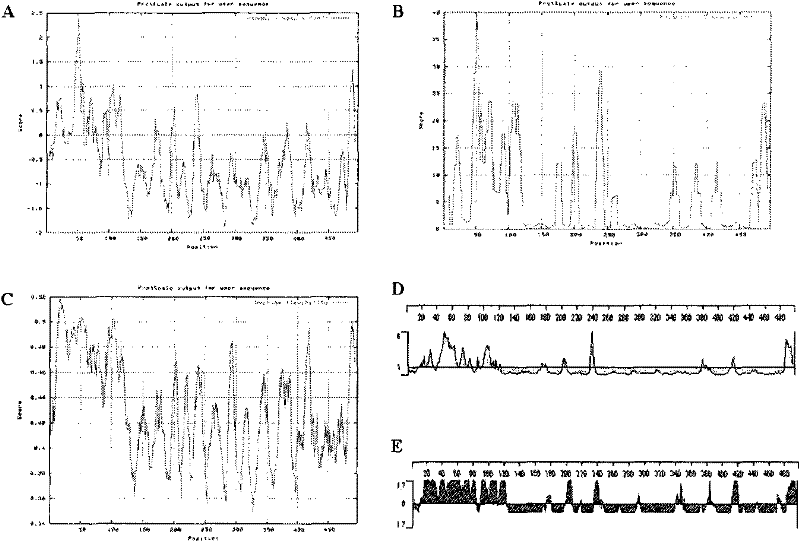
[0110] Obtain the EBV latent membrane protein 2 (LMP2) gene sequence and amino acid sequence from the network resource database (Genbank, Swiss-Prot); use the online network resource (EXPASY) and the biological software DNASTAR to analyze the antigenicity and surface accessibility of the EBV-LMP2 amino acid sequence The parameters such as property, flexibility and transmembrane region were analyzed ( figure 1 ), supplemented by the analysis results of the secondary structure of EBV-LMP2 protein, analysis and comparison of various parameters to comprehensively judge the B cell dominant epitope of EBV-LMP2.
[0111] After the above-mentioned epitope prediction, combined with repeated tests and selections by the inventors, the results obtained three amino acid sequences derived from EBV latent membrane protein 2, w...
Embodiment 2
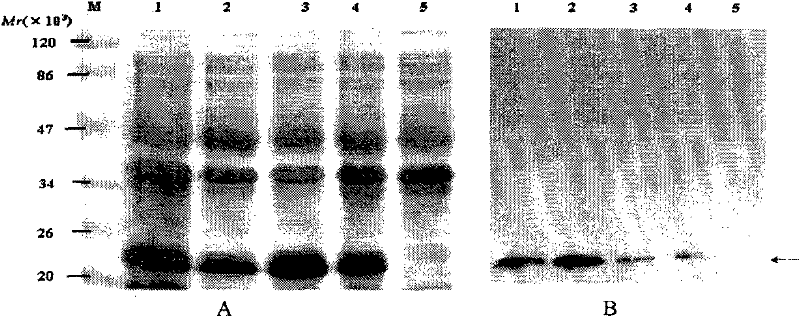
[0117] Example 2. Immunogenicity Study of EBV-LMP2B Cell Epitope Recombinant Protein
[0118] Female BALB / c mice aged 6-8 weeks were randomly divided into 5 groups, with 9 mice in each group: Groups 1-3 were the EBV-LMP2B cell epitope protein immunization group, and group 4 was the His-tag protein control group . B cell epitope protein and Freund's adjuvant (FCA) 1:1 (W / W) were fully emulsified evenly, and at 0, 2, and 4 weeks, 50 μg of B cell epitope protein or His-tag protein per mouse, Mice were immunized subcutaneously on the back. The humoral immune effect of the immunized mice was detected, that is, the titer and maintenance time of specific serum IgG.
[0119] At 0, 3, and 6 weeks, each group of mice was blooded by docking the tail to detect the level of serum IgG antibody by ELISA method.
[0120] 1. Determination results of serum-specific IgG antibodies of immunized mice
[0121] The three B cell epitope proteins of purified EBV-LMP2 were coated on the ELISA react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com