Preparation of doped poly-2,3-dimethylaniline and its application in anticorrosion coatings
A technology of dimethylaniline and basic coatings, which can be used in anti-corrosion coatings, polyester coatings, polyurea/polyurethane coatings, etc., and can solve the problems of doped P2,3-DMA that have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
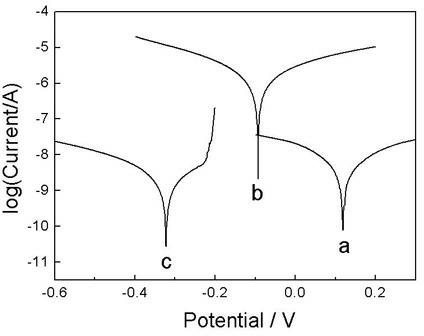
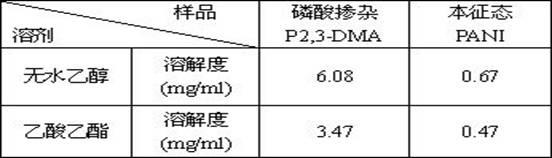
Image
Examples
Embodiment 1
[0035] In a water bath at 10°C, add 50ml of distilled water, 8.2×10 -3 mol sulfuric acid and 1.6×10 -2 mol 2,3-dimethylaniline, stir to mix evenly; dissolve 7.5g of ammonium persulfate in 25ml of distilled water, slowly drop into the reaction system, and react for 9 hours under stirring; after the reaction, suction filter, and distilled water Wash the filter cake until the filtrate is colorless; after drying the filter cake in an oven at 60°C for 6 hours, take it out and grind it to obtain sulfuric acid-doped P2,3-DMA powder.
[0036] The molecular weight was measured by high performance liquid chromatography.
[0037] The average molecular weight (weight average molecular weight) was 35428.
Embodiment 2
[0039] In a water bath at 40°C, add 50ml of distilled water, 2.5×10 -3 mol dodecylbenzenesulfonic acid and 1.6×10 -2 mol 2,3-dimethylaniline, stir to mix evenly; dissolve 7.5g of ammonium persulfate in 25ml of distilled water, slowly drop into the reaction system, and react for 9 hours under stirring; after the reaction, suction filter, and distilled water The filter cake was washed until the filtrate was colorless; after the filter cake was dried in an oven at 60°C for 8 hours, it was taken out and ground to obtain dodecylbenzenesulfonic acid-doped P2,3-DMA powder.
Embodiment 3
[0041] In a water bath at 70°C, add 50ml of distilled water, 4×10 -2 mol camphorsulfonic acid and 1.6×10 -2 mol 2,3-dimethylaniline, stir to mix evenly; dissolve 13g of ammonium persulfate in 25ml of distilled water, slowly drop into the reaction system, and react for 12 hours under stirring; after the reaction, suction filter and wash with distilled water Filter the cake until the filtrate is colorless; after drying the filter cake in an oven at 60°C for 10 hours, take it out and grind it to obtain P2,3-DMA powder doped with camphorsulfonic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com