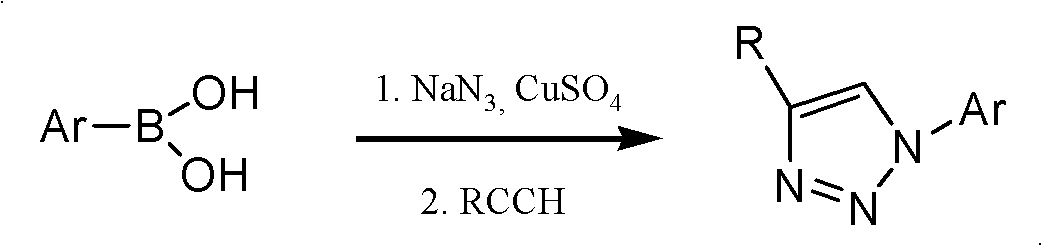
Preparation method of 2-deoxy-beta-D-glucopyranosyl triazole compound
A technology of glucopyranose and base triazole, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., and achieves the effects of green environmental protection yield, mild reaction conditions, and simple reaction operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Add 1,2-deoxy-3,4,6-tri-O-benzyl-7-C-acetyl-α-D-glucopyranose (47.2mg, 0.1mmol) to a 10mL reaction flask And sodium azide (9.8mg, 0.15mmol), then add PEG-400 (1mL) to dissolve, and stir at 80°C for 10h. Phenylacetylene (13.5 μL, 0.12 mmol) and CuI (1.9 mg) were then added at room temperature. Stir at room temperature, and TLC detects that the reaction is complete. Ethyl acetate was added for extraction (5mL×3), and the extract was concentrated and purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=3:1~2:1) to obtain 1-(2′-C- Acetylmethyl-2'-deoxy-3',4',6'-tri-O-benzyl-β-D-glucopyranose)-4-phenyl-1,2,3-triazole 50.6mg, Yield 82%. (c 0.1, CHCl 3 ); mp: 137-138°C; 1 H NMR (600MHz, CDCl 3 ):δ H 7.98(s, 1H), 7.86(d, J=7.2Hz, 2H), 7.46(t, J=7.7Hz, 2H), 7.41-7.22(m, 17H), 5.93(d, J=10.2Hz, 1H ), 5.00 (d, J = 11.6Hz, 1H), 4.87 (d, J = 10.8Hz, 1H), 4.70 (d, J = 10.8Hz, 1H), 4.64 (dd, J = 11.9, 4.6Hz, 2H ), 4.57(d, J=12.2Hz, ...
Embodiment 2
[0032]Example 2: Add 1,2-deoxy-3,4,6-tri-O-benzyl-7-C-acetyl-α-D-glucopyranose (47.2mg, 0.1mmol) to a 10mL reaction flask And sodium azide (9.8mg, 0.15mmol), then add PEG-400 (1mL) to dissolve, and stir at 80°C for 10h. Then, 2-methylphenylacetylene (15.0 μL, 0.12 mmol) and CuI (1.9 mg) were added at room temperature. Stir at room temperature, and TLC detects that the reaction is complete. Ethyl acetate was added for extraction (5mL×3), and the extract was concentrated and purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=3:1~2:1) to obtain 1-(2′-C- Acetylmethyl-2'-deoxy-3',4',6'-tri-O-benzyl-β-D-glucopyranose)-4-(2'-methylphenyl)-1,2, 3-triazole 48.0 mg, yield 76%. (c 0.3, CHCl 3 ); mp: 115°C; 1 H NMR (600MHz, CDCl 3 ):δ H 7.84(s, 1H), 7.78-7.72(m, 1H), 7.38-7.19(m, 19H), 5.87(d, J=10.2Hz, 1H), 4.98(d, J=11.6Hz, 1H), 4.84 (d, J=10.8Hz, 1H), 4.68(d, J=10.8Hz, 1H), 4.65-4.58(m, 2H), 4.54(d, J=12.2Hz, 1H), 3.88(t, J= 9.0Hz, 1H), 3.8...
Embodiment 3
[0033] Example 3: Add 1,2-deoxy-3,4,6-tri-O-benzyl-7-C-acetyl-α-D-glucopyranose (47.2mg, 0.1mmol) to a 10mL reaction flask And sodium azide (9.8mg, 0.15mmol), then add PEG-400 (1mL) to dissolve, and stir at 80°C for 10h. Then, 3-chlorophenylacetylene (14.3 μL, 0.12 mmol) and CuI (1.9 mg) were added at room temperature. Stir at room temperature, and TLC detects that the reaction is complete. Ethyl acetate was added for extraction (5mL×3), and the extract was concentrated and purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=3:1~2:1) to obtain 1-(2′-C- Acetylmethyl-2′-deoxy-3′,4′,6′-tri-O-benzyl-β-D-glucopyranose)-4-(3″-chlorophenyl)-1,2,3 - Triazole 48.8 mg, yield 75%. (c 0.4, CHCl 3 ); mp: 159°C; 1 H NMR (600MHz, CDCl 3 ):δ H 7.94(s, 1H), 7.84(s, 1H), 7.70(d, J=7.5Hz, 1H), 7.38-7.18(m, 17H), 5.88(d, J=10.2Hz, 1H), 4.97(d , J=11.6Hz, 1H), 4.84(d, J=10.8Hz, 1H), 4.67(d, J=10.8Hz, 1H), 4.61(dd, J=11.9, 6.3Hz, 2H), 4.54(d , J=12.2Hz, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com