A kind of preparation method of calcium carbonate particle
A technology of calcium carbonate and particles, applied in the direction of calcium carbonate/strontium/barium, etc., to achieve the effects of broad application prospects, low price, and simple and easy-to-operate preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
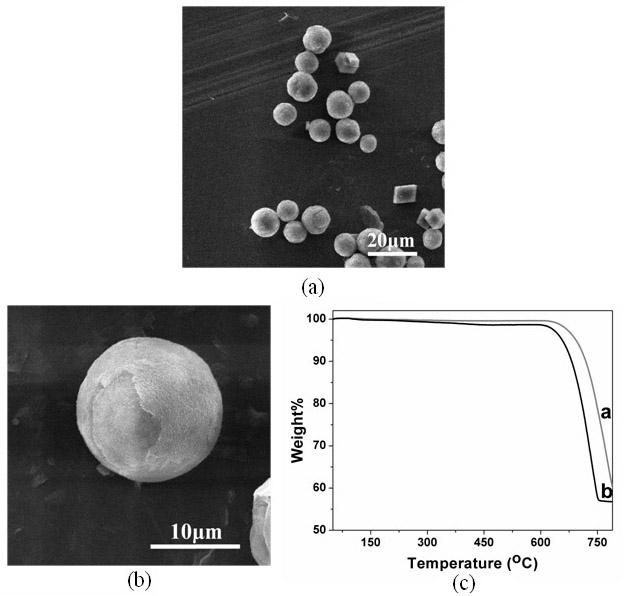
[0043] All glass instruments used in the experiment were pretreated as follows: firstly, they were sonicated in ethanol for 30 minutes, and then they were taken out and rinsed with deionized water. Soak in a mixed solvent of concentrated nitric acid / hydrogen peroxide / water at a volume ratio of 1:1:1 for 2 days, take it out, wash it with acetone, and dry it at room temperature. Prepare 100 ml of 0.0025 g / L GO aqueous solution, then add a certain amount of calcium chloride to prepare a 0.01 mol / L calcium chloride solution, and adjust the pH of the solution to 7.5. Cover the beaker containing the solution with a polyethylene film, and make 12 pinholes on the film. Take 20 grams of ammonium carbonate, grind it to a fineness of 100 mesh powder, place it in a 50 ml beaker, cover the mouth of the beaker with a polyethylene film, and prick 25 holes with a needle. Then put it into a desiccator with a volume of about 10 liters together with the above-mentioned beaker containing the sol...
Embodiment 2
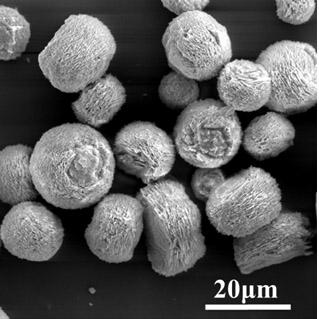
[0045] The glass instrument processing method is the same as in Example 1. Prepare 100 ml of 0.025 g / L GO aqueous solution, then add a certain amount of calcium chloride to prepare 0.01 mol / L calcium chloride solution, and adjust the pH of the solution to 7.5. Other follow-up operations are all the same as in Example 1, except that the reaction time is extended to 4 days. The resulting calcium carbonate particles see figure 2 , which is also a microsphere with a core-shell structure, with a size between 15-20 microns. However, compared with the microspheres obtained in Example 1, the surface is more crisp and rough, and the gaps between the formed sheets can be clearly seen.
Embodiment 3
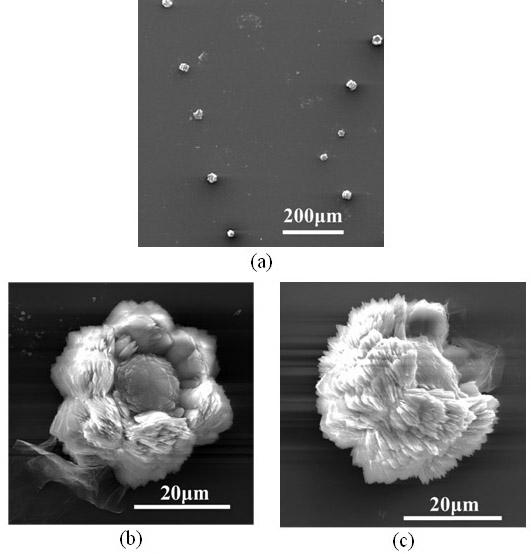
[0047] The glass instrument processing method is the same as in Example 1. Prepare 100 milliliters of 0.0025 g / L GO aqueous solution, add a certain amount of calcium chloride to prepare 0.001 mol / L calcium chloride solution, adjust the pH of the solution to 8, cover the beaker containing the solution with a polyethylene film, the film Make 8 pinholes on the top. Take 20 grams of ammonium carbonate, grind it to a powder with a fineness of 100 mesh, put it in a 50 ml beaker, cover the mouth of the beaker with a polyethylene film, and prick 20 holes with a needle. Then put it into a desiccator with a volume of about 10 liters together with the above-mentioned beaker containing the solution, seal it, and start the reaction at a temperature of 30±1°C. After reacting for 5 hours, the resulting suspension was centrifuged, washed with water and centrifuged three times alternately. The obtained powder was dried at 40° C. for 12 hours under a vacuum of about 10 Pa. Get the desired pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com