Fluorescent material emitting red and green light excited by blue light and preparation method thereof
A blue light excitation and fluorescent material technology, applied in the field of luminescent materials, can solve the problems of mixed white light chromatic aberration, complicated power supply, low color rendering index, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
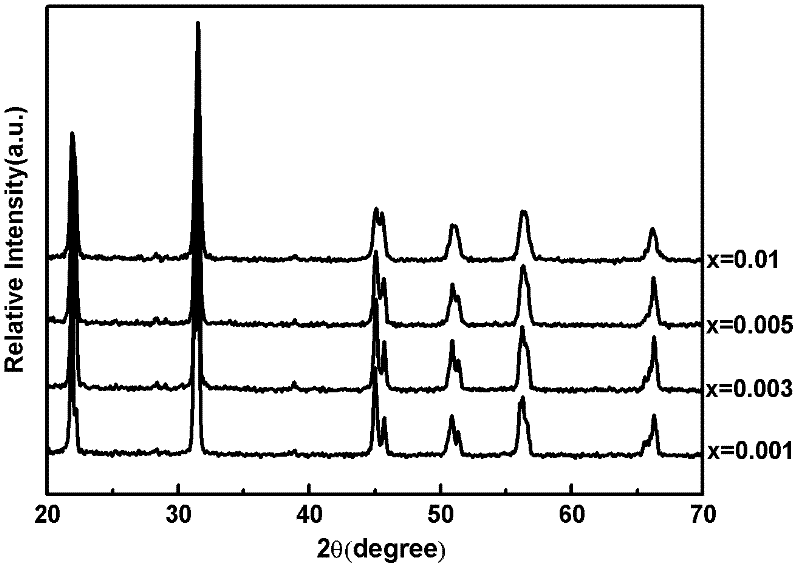
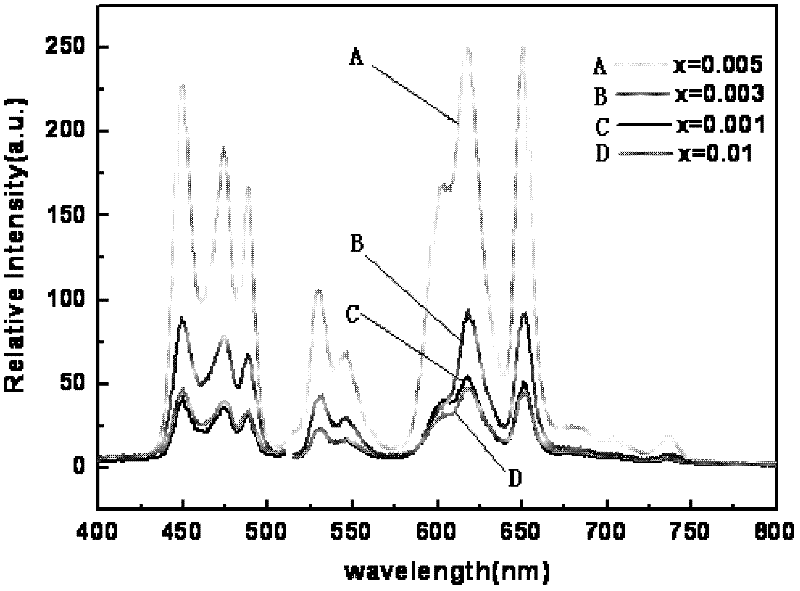
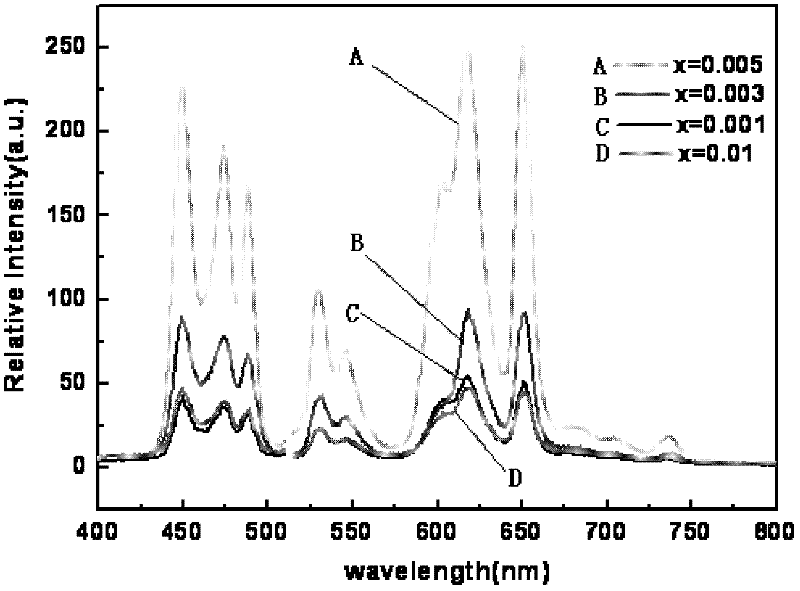
[0041] Preparation (Na 0.5 K 0.5 ) 1-x PR x NbO 3 Fluorescent material, wherein the values of x are respectively x=0.001, 0.003, 0.005, and 0.01.
[0042] Select raw material NaCO 3 、KCO 3 、Pr 6 o 11 and Nb 2 o 5 , according to (Na 0.5 K 0.5 ) 1-x PR x NbO 3 (x=0.001, 0.003, 0.005, 0.01) in the stoichiometric ratio of the corresponding elements Weigh the selected raw materials respectively, mix the corresponding raw materials, put them into the ball mill tank, add absolute ethanol and zirconia balls in the ball mill After ball milling for 24 hours, discharge and dry with absolute ethanol to obtain ball mill powder, wherein the mass ratio of absolute ethanol to raw material mixture is 3:1, and the mass ratio of zirconia balls to raw material mixture is 1.5:1. The ball mill powder was pre-fired in an alumina crucible, the pre-fire temperature was 880°C, and the heating rate was 3°C / min. Water ethanol and zirconia balls are ball-milled for 24 hours and dried, th...
Embodiment 2
[0047] Preparation (Na 0.6 K 0.4 Li 0.1 ) 0.995 PR 0.005 NbO 3 : 0.05K fluorescent material.
[0048] Select raw material Na 2 CO 3 、K 2 CO 3 、Pr 6 o 11 , Nb 2 o 5 and Li 2 CO 3 , according to (Na 0.6 K 0.4 Li 0.1 ) 0.995 PR 0.005 NbO 3 : The stoichiometric ratio of the corresponding elements in 0.05K weighs the selected raw materials respectively, mixes the corresponding raw materials that have been weighed, puts them into a ball mill jar, adds absolute ethanol and zirconia balls, and ball mills them on the ball mill for 24 hours, then use The water and ethanol are discharged and dried to obtain ball mill powder, wherein the mass ratio of absolute ethanol to the raw material mixture is 2:1, and the mass ratio of zirconia balls to the raw material mixture is 1:1. Put the ball mill powder in an alumina crucible for pre-burning, the pre-burning temperature is 800°C, the heating rate is 3°C / min, keep it warm for 1 hour and cool naturally to get the pre-fired ...
Embodiment 3
[0053] Preparation (Na 0.6 K 0.01 Na 0.49 ) 0.995 PR 0.005 NbO 3 : 0.1Na fluorescent material.
[0054] Select raw material Na 2 CO 3 、K 2 CO 3 、Pr 6 o 11 and Nb 2 o 5 , according to (Na 0.6 K 0.01 Na 0.49 ) 0.995 PR 0.005 NbO 3 : The stoichiometric ratio of the corresponding elements in 0.1Na is respectively weighed the selected raw materials, after the corresponding raw materials weighed are mixed, put into the ball mill tank, add absolute ethanol and zirconia balls and ball mill on the ball mill for 24 hours, then use The water and ethanol are discharged and dried to obtain ball mill powder, wherein the mass ratio of absolute ethanol to the raw material mixture is 1:1, and the mass ratio of zirconia balls to the raw material mixture is 1.5:1. Put the ball mill powder in an alumina crucible for pre-burning, the pre-burning temperature is 900°C, the heating rate is 3°C / min, keep warm for 6 hours and cool naturally to get the pre-fired powder, grind the pre-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com