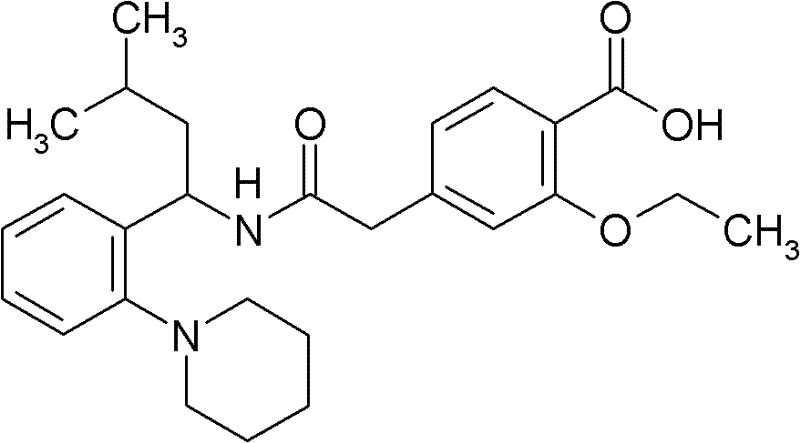
A repaglinide crystal, its preparation method and solid oral preparation containing the crystal
A technology of oral preparations and crystals, which is applied in the field of medicine, can solve the problems of poor bioavailability and low solubility of repaglinide, and achieve the effect of convenient recycling, convenient separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] [Example 1] Preparation of repaglinide crystals
[0080] 1) Dissolve repaglinide powder in ethanol to prepare a repaglinide solution as the dispersed phase;
[0081] 2) Dissolve sodium 2-ethylhexyl succinate sulfonate in isooctane to prepare a sodium 2-ethylhexyl succinate sulfonate solution as a dispersion medium;
[0082] 3) Under an ultrasonic field, use a micro-injector to add the dispersed phase solution to the dispersion medium, continue to sonicate, and centrifuge to separate repaglinide ultrapowder, remove the supernatant and add isooctane to wash and centrifuge to remove Sodium 2-ethylhexyl succinate sulfonate adsorbed on the surface of repaglinide is dried in vacuum to obtain repaglinide crystals.
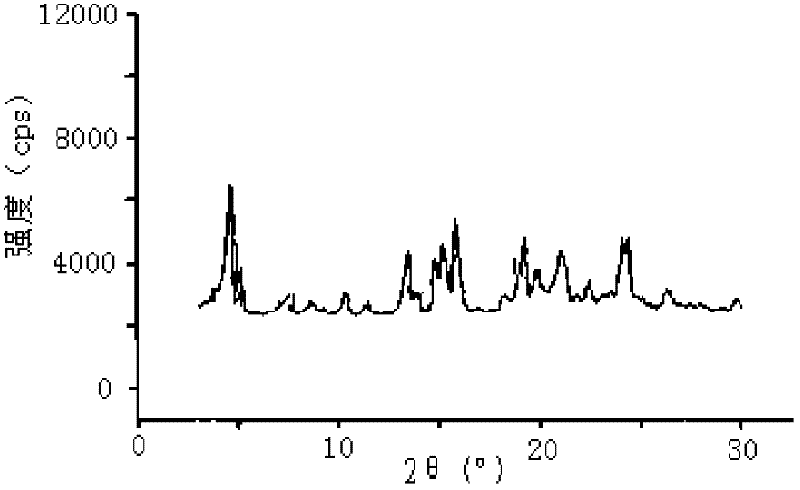
[0083] The particle size of the prepared repaglinide crystal is 1μm, and the X-ray powder diffraction pattern obtained by Cu-Kα ray measurement (see figure 1 The characteristic peaks in) are displayed at 2θ of 4.8°, 13.6°, 14.9°, 15.2°, 16.0°, 19.1°, 21.2° and 24.1°.
Embodiment 2
[0084] [Example 2] Preparation of repaglinide crystals
[0085] 1) Dissolve repaglinide powder in ethanol to prepare a repaglinide solution as the dispersed phase;
[0086] 2) Dissolve sodium 2-ethylhexyl succinate sulfonate in isooctane to make 0.05 mol·L -1 Sodium 2-ethylhexyl succinate sulfonate solution as the dispersion medium;
[0087] 3) In an ultrasonic field with a power of 0.1KW, use a micro-injector to add the dispersed phase solution to the dispersion medium, and continue to sonicate for 1 minute at 9000r·min -1 Repaglinide ultra-powder was separated by high-speed centrifugation under the conditions of high-speed centrifugation, and the supernatant was removed and isooctane washed and centrifuged twice to remove the sodium 2-ethylhexyl succinate sulfonate adsorbed on the surface of repaglinide. Vacuum drying for 8 hours, the repaglinide crystal is obtained.
[0088] The particle size of the obtained repaglinide crystal was 5 μm, and the X-ray powder diffraction pattern mea...
Embodiment 3
[0089] [Example 3] Preparation of repaglinide crystals
[0090] 1) Dissolve repaglinide powder in ethanol to prepare a repaglinide solution as the dispersed phase;
[0091] 2) Dissolve sodium 2-ethylhexyl succinate sulfonate in isooctane to prepare 0.3 mol·L -1 Sodium 2-ethylhexyl succinate sulfonate solution is used as the dispersion medium;
[0092] 3) In an ultrasonic field with a power of 0.3KW, add the dispersed phase solution to the dispersion medium with a micro-injector, and continue to sonicate for 3 minutes at 11000r·min -1 Repaglinide ultra-powder was separated by high-speed centrifugation under high-speed conditions, and the supernatant was removed and isooctane washed and centrifuged 4 times to remove the sodium 2-ethylhexyl succinate sulfonate adsorbed on the surface of repaglinide. After vacuum drying for 12 hours, repaglinide crystals were obtained.
[0093] The particle size of the obtained repaglinide crystal was 3 μm, and the X-ray powder diffraction pattern obtaine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com