Chemical conjugate of recombinant deleted human keratinocyte growth factor type I
A technology of keratinocytes and growth factors, applied in the field of genetic engineering, to achieve the effect of protecting acute small intestinal mucosal injury and acute liver injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0031] Example 1. Recombinant expression of human keratinocyte growth factor type I (Δ23hKGF) lacking 23 amino acids at the N-terminal
[0032] According to the Δ23hKGF amino acid sequence (SEQ ID No: 1), the corresponding cDNA sequence of the E. coli preferred codon was designed to include the 5'end and 3'respectively to design the restriction enzyme Nde I and BamH I sites (SEQ ID No: 2) . Entrusted Bao Bioengineering (Dalian) Co., Ltd. to artificially synthesize the entire gene and insert it into the pUC18 vector and named it pUC18-Δ23hKGF / DH5α.
[0033] Plasmid pUC18-Δ23hKGF DH5α and expression vector plasmid pET-3c were digested with restriction enzymes Nde I and BamH I. According to the results of 1% agarose electrophoresis, a fragment of about 4638 bp after digestion with pET-3c was recovered, and pUC18- After Δ23hKGF / DH5α is a fragment of about 450 bp after digestion, connect the two fragments with T4 ligase, use LA (tryptone 2g, yeast extract 1g, NaCl 2g, agar 3g, add wate...
example 2
[0035] Example 2. Purification and preparation of Δ23hKGF
[0036] Take out the bacteria from the refrigerator at -20℃, and put in the bacterial lysate (50mmol / L Tris-HCl, 5mmol / LEDTA, 10μg / ml DNase, 2mm MgCl at 1:15w / v) 2 , PH 8.5-9.0), add trisodium citrate with a final concentration of 0.1M when stirring at 37°C until the cleavage solution is not viscous, and continue stirring for 0.5h. Adjust the pH to 7.5, centrifuge at 10,000 rpm for 15 minutes, and collect the supernatant. The supernatant was applied to an SP-Sepharose Fast Flow column (equilibration solution: 50mmpb, pH7.5), re-equilibrated, and eluted with a linear gradient of 50-500mm Nacl. The elution peaks were collected and detected by SDS-PAGE to determine the main target protein. Elution peak. The target sample eluted by SP was applied to a Heparin column (equilibration solution: 20mm pb, pH 7.5), re-equilibrated, and eluted with a linear gradient of 50-500mm Nacl. The elution peaks were collected and detected by ...
example 3
[0037] Example 3: mPEG-MAL-20K modification of Δ23hKGF and purification of modified (PEG-20K-Δ23KGF)
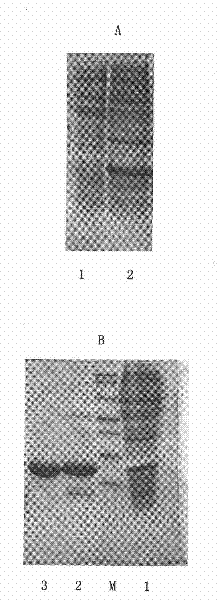
[0038] For Δ23hKGF solution with a purity greater than 95%, use 100mmol / L phosphate buffer, pH6.5, dilute the sample to 2mg / mL, and press Δ23hKGF and mPEG-MAL-20KD (linear type, purchased from Beijing Jiankai Technology Co., Ltd.) The ratio is 1:2. Weigh mPEG-MAL-20KD and add it to the Δ23hKGF solution, stir the reaction for 2hr at 25°C and 100r / min, and stop the reaction by lowering the pH with hydrochloric acid. The modified Δ23hKGF (PEG-20K-Δ23KGF) solution was dialyzed overnight (dialysis fluid: 20mmNaAc, pH5.0), and the dialyzed PEG-20K-Δ23KGF solution was applied to an SP-Sepharose Fast Flow column (equilibration solution: 20mmNaAc, pH5.0) ), re-equilibration, eluted with a linear gradient of 50-500mm Nacl, collected the elution peak, and was detected by SDS-PAGE and HPLC to obtain PEG-20K-Δ23KGF ( figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com