Method for purifying human immunoglobulin from separated component I+III of blood plasma
A human immunoglobulin and plasma separation technology, applied in the field of separation and purification of human immunoglobulin, can solve the problems of retaining the physicochemical properties and biological activity of the immunoglobulin, unable to completely inactivate/remove the virus, and the purification effect is difficult to meet the requirements. , to achieve the effect of shortening the production process time, good economic and social value, and considerable economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
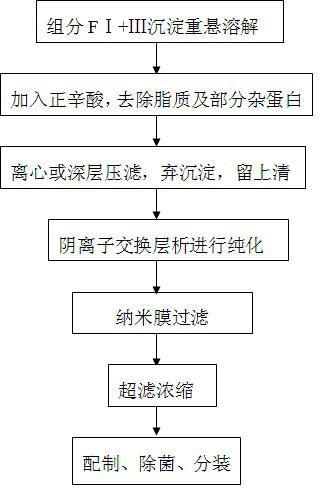
[0049] The present invention is realized by the following steps:
[0050] a. Dissolution of component I+III precipitate:
[0051] Preparation of acetic acid sodium acetate buffer: Take 6.9 mL of glacial acetic acid and 10.88 g of sodium acetate to prepare 10 L of acetic acid sodium acetate buffer with a content of 0.02 mol / L, and adjust the pH to 4.5;
[0052] Fully dissolve the component I+III precipitate with 3 times the weight of acetic acid sodium acetate buffer at 7°C;
[0053] b. After the sample is fully dissolved, heat up to 20°C, add 0.3mol / L NaOH solution at a flow rate of 0.6L / min, adjust the pH to 4.5±0.05, stir for 1 hour, and then add positive Octanoic acid, so that the n-octanoic acid in the solution can be evenly distributed until the final concentration of octanoic acid in the solution is 100mmol / L, stop adding octanoic acid, stir vigorously to facilitate the thorough mixing of octanoic acid, stir for 2 hours, precipitate non-IgG miscellaneous proteins, and...
Embodiment 2
[0065] The specific implementation steps of the process of the present invention are as follows:
[0066] a. Component I + III precipitates were dissolved in 0.02mol / L acetic acid sodium acetate buffer at 7°C with 3 times the amount of precipitation, and fully dissolved at room temperature;
[0067] b. After fully dissolving the sample, do not adjust the pH, and raise the temperature to 20°C. After fully stirring and mixing, slowly add n-octanoic acid to make the final concentration of octanoic acid in the solution 110mmol / L, and vigorously stir to facilitate the thorough mixing of octanoic acid. Stir for 2 Hour;
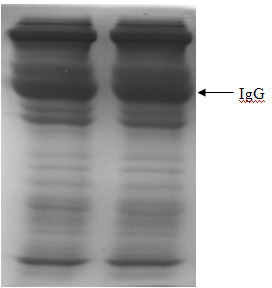
[0068] c. Rinse the filter plate of the filter press with 600 kg of water for injection at 20°C at a flow rate of 20 kg / min, then dry the filter plate with compressed air at normal temperature, press filter after balancing, and obtain a clarified filtrate, in which the IgG purity is 65%. The above; adjust the pH of the above filtrate to 5.8 with 0.3mol / L NaOH, fi...
Embodiment 3
[0075] The specific implementation steps of the process of the present invention are as follows:
[0076] In step a, the precipitate of component I+III is dissolved in 3 times the amount of precipitate, pH 4.5, concentration of 0.02mol / L acetic acid sodium acetate buffer, fully dissolved at room temperature;
[0077] In step b, n-octanoic acid is slowly added to make the final concentration of octanoic acid in the solution 90mmol / L. Through this step, the IgG purity in the solution reaches 65%, and the lipid-enveloped virus is removed;
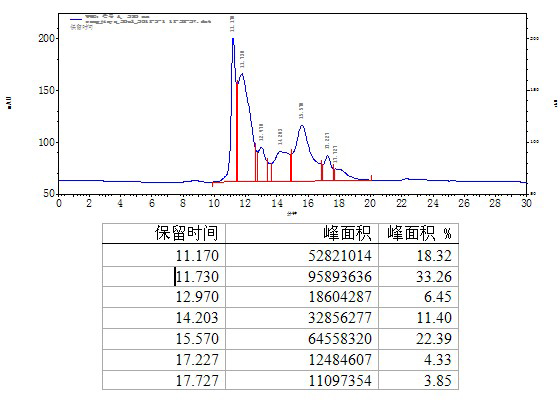
[0078] In step c, use 600kg of water for injection at 20°C to wash the filter plate of the filter press at a flow rate of 20kg / min, then dry the filter plate with compressed air at normal temperature, press filter after balancing, and obtain a clarified filtrate; use 0.3mol / min of the filtrate Adjust the pH to 5.4 with L NaOH; filter with a 0.45um filter, adjust the conductivity of the solution to 1.5mS / cm, and purify on an anion exchange chro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com