Solid-phase nano micelle and preparation method thereof
A technology of nanomicelles and drug-loaded micelles, which can be used in pharmaceutical formulations, medical preparations of non-active ingredients, non-effective ingredients of polymer compounds, etc., and can solve problems such as limited diffusion free volume, large molecular weight, and unsatisfactory effects. , to achieve the effect of improving bioavailability, high cohesion, and prolonging the circulation time in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
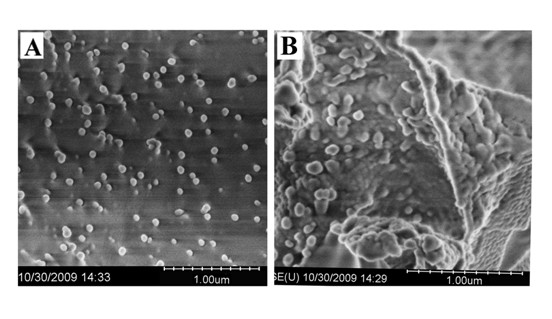
[0039] Embodiment 1: Preparation of solid phase nano micelles and observation of product particle size and morphology.
[0040] Polyoxyethylene 600 mono / distearate, Polyoxyethylene 400 monostearate, Benze 56, Benze 76, Benze 78, Benze 97, Lipocol C-10, Volpo S-10, Lipocol S - 10 or their mixture (0.2-5g), dissolved in 100ml aqueous solution containing 1-5 (w / v%) lactose, the water temperature is kept at 50°C. Model drug furosemide 20mg, naproxen 80mg or paclitaxel 10mg were dissolved in 5ml ethyl acetate. The drug solution is added dropwise to the surfactant solution, the water temperature is kept at 50° C., the organic solvent is removed by stirring or rotary evaporation, and a clear drug-loaded micellar solution is obtained. Spray drying (parameters: the inlet temperature is lower than the bluish point of the micellar solution; spray speed: 1ml / min; spray drying air pressure: 1.5kgf / cm 2 ;Air velocity: >0.25m 3 / min), to obtain dry powder.
[0041] The particle size and ...
Embodiment 2
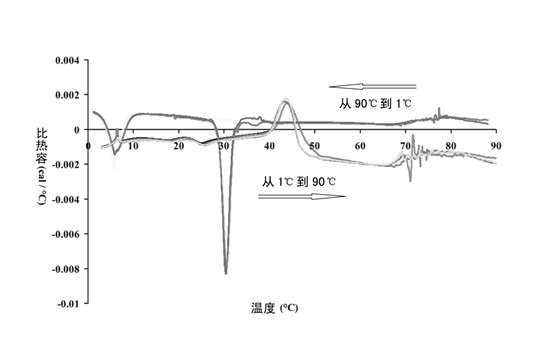
[0044] Example 2: Preparation of solid-phase nanomicelles and their in vivo and in vitro evaluation as poorly soluble drug delivery carriers.
[0045] Polyoxyethylene 600 mono / distearate, Polyoxyethylene 400 monostearate, Benze 56, Benze 76, Benze 78, Benze 97, Lipocol C-10, Volpo S-10, Lipocol S -10 or their mixture (0.2-5g) and model drug furosemide 10-500mg, naproxen 10-500mg or paclitaxel 10-100mg are dissolved in 10ml of ethanol. The solution is added dropwise to 100 ml of an aqueous solution containing 1-10 (w / v%) mannitol, and the water temperature is maintained at 50° C. The organic solvent was removed by stirring or rotary evaporation to obtain a clear drug-loaded micellar solution. Spray drying (parameters: inlet temperature is lower than the cloud point of micellar solution; spray speed: 1ml / min; spray drying air pressure: 1.5kgf / cm2; air flow rate:> 0.25m3 / min) to obtain dry powder.
[0046] In vivo and in vitro evaluation of solid-phase nanomicelles as poorly so...
Embodiment 3
[0052] Example 3: The formulation of paclitaxel-loaded solid-phase nanomicelles improves the oral bioavailability of the drug.
[0053] Dissolve Benzaze 76 (1g) and Paclitaxel 10-100mg in 10ml of ethyl acetate, remove the organic solvent by rotary evaporation, add 100ml of aqueous solution containing 1-5 (w / v, %) dextran, keep the water temperature at 50°C, stir to obtain clarification drug-loaded micellar solution. Spray drying (parameters: the inlet temperature is lower than the bluish point of the micellar solution; spray speed: 1ml / min; spray drying air pressure: 1.5kgf / cm 2 ;Air velocity: >0.25m 3 / min) to obtain a dry powder.
[0054] The paclitaxel-loaded solid-phase nanomicelle preparation was reconstituted with water to form a paclitaxel suspension containing 1 mg / ml, and administered to SD rats by intragastric administration (dose: 2.67 mg / kg). , 2, 4, 8, 12, and 24 hours later, blood was taken from the orbital vein, anticoagulated with heparin, centrifuged at 3,0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com