Recombinant human endostatin sustained-release injection oil preparation and preparation process thereof
A technology of vascular endothelium and injection oil, which is applied in the direction of drug combination, oil/fat/wax non-active ingredients, medical preparations of non-active ingredients, etc. It can solve the problems of complicated process, difficult to obtain, expensive and other problems, and achieve the technical process Convenience, reduced cost of medication, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] prescription:
[0039] Endostar 40g
[0040] Aluminum monostearate 5g
[0041] Soybean oil for injection about 1000ml
[0042] Make a total of 1000ml
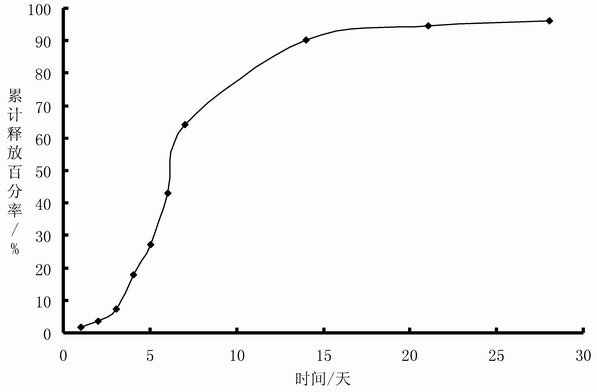
[0043] Preparation: Prepare Endostar with water for injection into a solution with a concentration of 2%, freeze-dry to obtain Endostar powder, and micronize the powder through a 300-mesh sieve; add Endostar to the monostearic acid-containing Aluminum 0.5% in the gelatinized soybean oil for injection, stir evenly to obtain the oil suspension sample containing Endostar, the proportion of solid particles in the sample whose particle size distribution is between 10 and 100 microns is 97.2%, and the sample viscosity is 0.236Pa·S;
[0044] Determination of release in vitro: 1 ml of the sample prepared above was put into a dialysis bag with a molecular weight cut-off of 300,000 and a side length of 1 cm, and the two ends were tightened with clips. The dialysis bag containing the sample was placed in a container with a mou...
Embodiment 2
[0046] prescription:
[0047] Endostar 40g
[0048] Aluminum monostearate 40g
[0049] Soybean oil for injection about 1000ml
[0050] Make a total of 1000ml
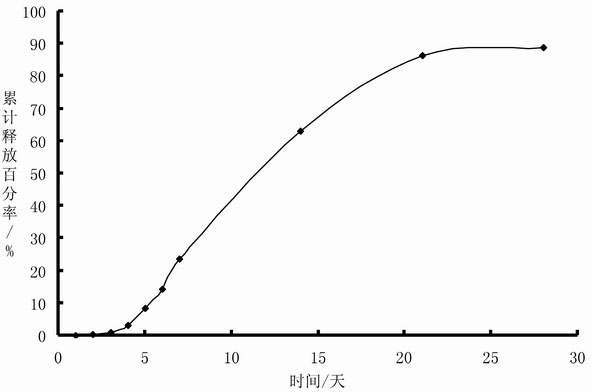
[0051] Preparation: Prepare Endostar with water for injection into a solution with a concentration of 2%, freeze-dry to obtain Endostar powder, and micronize the powder through a 300-mesh sieve; add Endostar particles to the stearin-containing Aluminate 4% soybean oil for injection gelled, stirred evenly to obtain an oil suspension sample containing Endostar, the proportion of solid particles in the sample whose particle size distribution was between 10 and 100 microns was 97.9%, and the sample Viscosity is 16.48Pa S; Sample is measured its in vitro release rate according to the method in embodiment 1, and measurement result sees embodiment 2 and figure 2 .
Embodiment 3
[0053] prescription:
[0054] Endostar 40g
[0055] Aluminum monostearate 10g
[0056] About 1000ml sesame oil for injection
[0057] Make a total of 1000ml
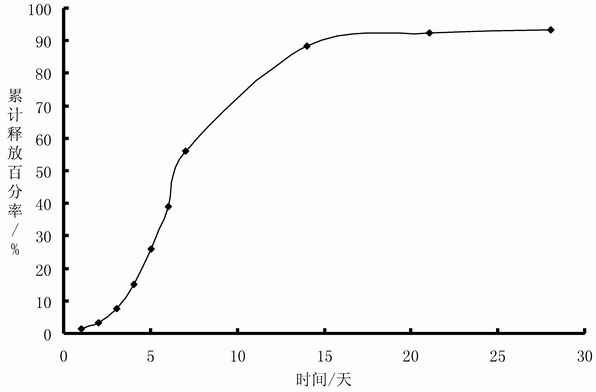
[0058] Preparation: Prepare Endostar with water for injection into a solution with a concentration of 2%, freeze-dry to obtain Endostar powder, and micronize the powder through a 300-mesh sieve; add Endostar particles to the stearin-containing Alumina 1% gelled sesame oil for injection, stirred evenly to obtain an oil suspension sample containing Endostar, the proportion of solid particles in the sample whose particle size distribution was between 10 and 100 microns was 96.2%, and the sample Viscosity is 0.248Pa S; Sample is measured its in vitro release according to the method described in embodiment 1, and measurement result sees embodiment 3 and image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com