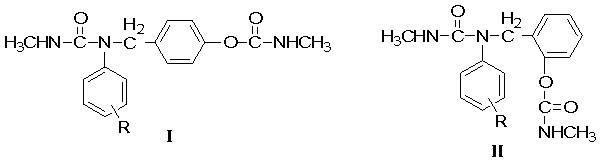
Urea carbamate methyl substituted arly ester with bactericidal activity
A technology of ureamethyl carbamate and bactericidal activity, which is applied in the field of pesticides to achieve the effects of easy-to-obtain raw materials, simple synthesis, and broadened biological activity spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0050] Example 1: Synthesis of 4-(N-methyl-N'-phenylureidomethyl)phenyl N-methylcarbamate.
[0051] 4-(anilinomethyl)phenol (1.99g, 0.01mol), Et 3 N (5.06mg, 0.05mmol) was dissolved in chloroform, stirred continuously at room temperature (about 30°C), slowly added methyl isocyanate (CH 3 NCO, 2.85g, 0.05mol), after adding dropwise for 10 minutes, continue to react at 60~65°C for 1 hour, stop the reaction, desolvate under reduced pressure to obtain a yellow oily substance, separate and purify by flash column chromatography to obtain a white solid Product, yield: 66.8%, melting point (mp): 151.2-153.1°C.
[0052] 1 H NMR (CDCl 3 , 500MHz) δ : 7.33~7.36(m, 2H), 7.27~7.29(m, 1H), 7.21~7.23(m, 2H), 7.07~7.09(m, 2H), 7.00~7.02(m, 2H), 4.84(s, 2H, CH 2 ), 2.95(s, 3H, CH 3 ), 2.75(s, 3H, CH 3 ). 13 C NMR (CDCl 3, 125MHz) δ : 157.73, 155.21, 150.05, 141.46, 135.59, 129.87(2C), 129.22(2C), 128.61(2C), 127.69(2C), 121.29, 52.48, 27.59, 27.44. IR (KBr) n: 3383, 3310, 3289, 29...
example 2
[0053] Example 2: Synthesis of 4-(N-methyl-N'-(4-methylphenyl)ureidomethyl)phenyl N-methylcarbamate.
[0054] 4-((4-methylanilino)methyl)phenol (2.13g, 0.01mol), Et 3 N (5.06mg, 0.05mmol) was dissolved in chloroform, stirred continuously at room temperature (about 30°C), slowly added methyl isocyanate (CH 3 NCO, 2.85g, 0.05mol), after adding dropwise for 10 minutes, continue to react at 60~65°C for 1 hour, stop the reaction, desolvate under reduced pressure to obtain a yellow oily substance, separate and purify by flash column chromatography to obtain a white solid Product, yield: 66.9%, melting point (mp): 143.1-144.7°C.
[0055] 1 H NMR (CDCl 3 , 500MHz) δ : 7.20~7.22(m, 2H), 7.11~7.13(m, 2H), 6.99~7.00(m, 2H), 6.92~6.94(m, 2H), 4.80(s, 2H, CH 2 ), 2.94(s, 3H, CH 3 ), 2.73(s, 3H, CH 3 ), 2.32(s, 3H, CH 3 ). 13 C NMR (CDCl 3, 125MHz) δ : 157.93, 155.26, 150.03, 138.65, 137.68, 135.67, 130.47(2C), 129.24(2C), 128.41(2C), 121.26(2C), 52.45, 27.57, 27.41, 20.96. IR ...
example 3
[0056] Example 3: Synthesis of 4-(N-methyl-N'-(4-methoxyphenyl)ureidomethyl)phenyl N-methylcarbamate.
[0057] 4-((4-Methoxyanilino)methyl)phenol (2.28g, 0.01mol), Et 3 N (5.06mg, 0.05mmol) was dissolved in chloroform, stirred continuously at room temperature (about 30°C), slowly added methyl isocyanate (CH 3 NCO, 2.85g, 0.05mol), after adding dropwise for 10 minutes, continue to react at 60~65°C for 1 hour, stop the reaction, desolvate under reduced pressure to obtain a yellow oily substance, separate and purify by flash column chromatography to obtain a white solid Product, yield: 69.6%, melting point (mp): 123.9-126.1°C.
[0058] 1 H NMR (CDCl 3 , 500MHz) δ : 7.21~7.23(m, 2H), 7.00~7.02(m, 2H), 6.95~6.97(m, 2H), 6.83~6.85(m, 2H), 4.79(s, 2H, CH 2 ), 3.79(s, 3H, OCH 3 ), 2.88(s, 3H, CH 3 ), 2.75(s, 3H, CH 3 ). 13 C NMR (CDCl 3, 125MHz) δ : 158.77, 158.09, 155.24, 150.05, 135.61, 133.74, 129.93(2C), 129.33(2C), 121.24(2C), 114.93(2C), 55.30, 52.53, 27.54, 27.40. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com