Continuous production method of propyl acetate
A technology of n-propyl acetate and n-propanol, which is applied in the field of continuous production of n-propyl acetate, can solve the problems of a large amount of organic solvents, high energy consumption, and a large amount of total waste liquid, and achieve simple preparation process and product selectivity Good, simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
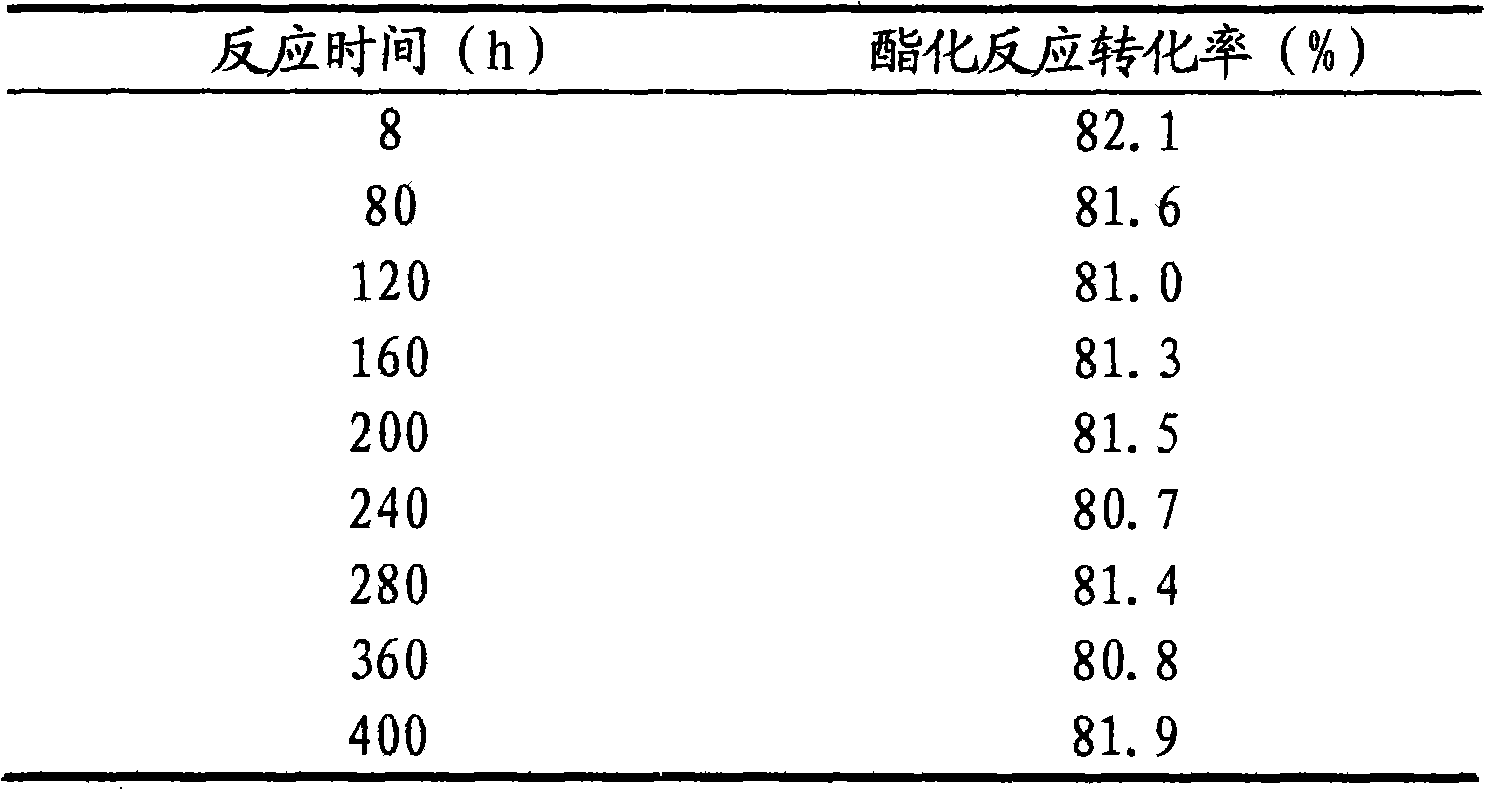
[0025] Attached figure 1 The process flow is carried out, in the figure 1-glacial acetic acid, 2-n-propanol, 3-catalyst, 4-esterification tank, 5-esterification tower, 6-layer device, 7-distributor,
[0026] Glacial acetic acid 1 100g, n-propanol 2 100g and FeCl 3 3.33 g with 1-methylimidazolium fluoroborate [Hmim] + BF4 - Add 16.67g of the prepared ionic liquid catalyst 3 into the esterification kettle 4 for heating and esterification reaction. The reaction temperature is 80°C. After the reaction starts to distill, continue to inject glacial acetic acid into the esterification kettle at a speed of 20g / h 100g of mixed raw materials with 100g of n-propanol is pumped into the esterification tank, the reaction product is separated in the esterification tower 5, and the unreacted raw materials are returned from the bottom of the esterification tower to continue the reaction in the esterification tank. The temperature at the top of the tower should be controlled at 80±10°C, the...
Embodiment 2
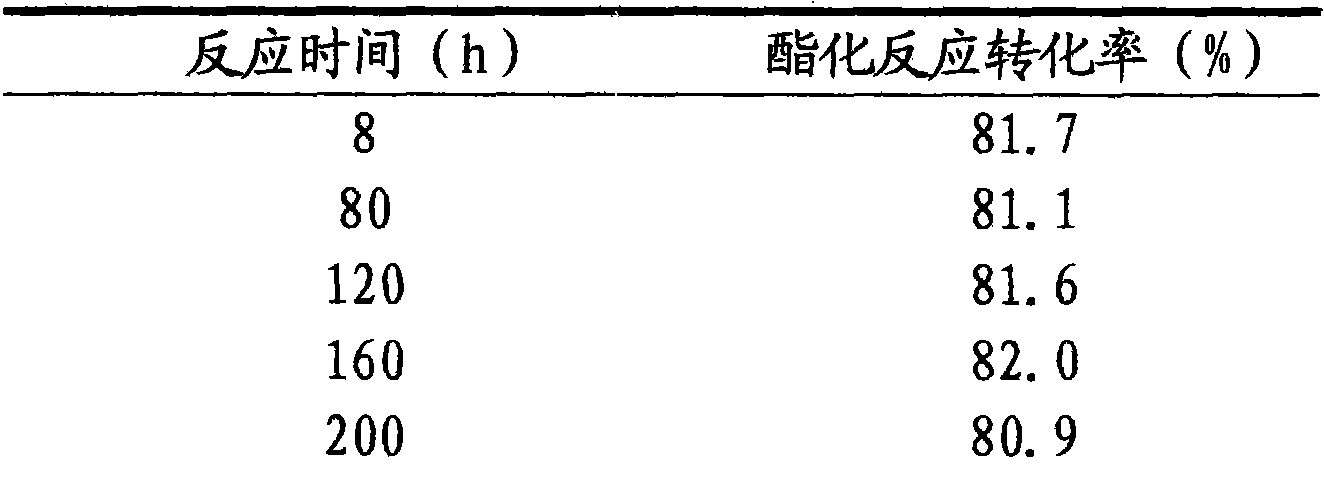
[0030] With the technical process of embodiment 1, glacial acetic acid 100g, n-propanol 110g, SnCl4 9.69 g and 1-ethylimidazolium fluoroborate [Heim] + BF4 - The ionic liquid catalyst that 32.31g makes is added in the esterification kettle and carries out heating esterification reaction, and reaction temperature is 85 ℃, after the reaction starts back distillation, then continuously inject glacial acetic acid 100g with the speed of 20g / h in the esterification kettle The mixed raw materials of n-propanol 110g ratio are pumped into the esterification tank, the reaction product is separated in the esterification tower, and the unreacted raw materials are returned from the bottom of the esterification tower to the esterification tank to continue the reaction, and the esterification tower top The temperature should be controlled at 80±10°C, the reflux ratio at the top of the esterification tower is 1:1.5, the acetic acid content of the distilled product at the top of the tower is ...
Embodiment 3
[0035] With the technological process of embodiment 1, glacial acetic acid 100g, n-propanol 110g, AlCl 3 16.33 g and 1-butylimidazolium fluoroborate [Hbim] + BF4 - The ionic liquid catalyst that 46.67g makes is added in the esterification kettle and carries out heating esterification reaction, and reaction temperature is 90 ℃, after the reaction starts back distillation, inject 100g of glacial acetic acid and 100g of glacial acetic acid and The mixed raw materials of n-propanol 110g ratio are pumped into the esterification tank, the reaction product is separated in the esterification tower, and the unreacted raw materials are returned from the bottom of the esterification tower to the esterification tank to continue the reaction, and the esterification tower top The temperature should be controlled at 80±10°C, the reflux ratio at the top of the esterification tower is 1:1.5, the acetic acid content of the distilled product at the top of the tower is less than 0.1%, and the n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com