Method for separating chromium ions and iron ions in multicomponent solution
A technology of chromium ions and iron ions, applied in the direction of chromium sulfate and the improvement of process efficiency, can solve the problems of long process flow and technical limitations, and achieve the effect of short process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
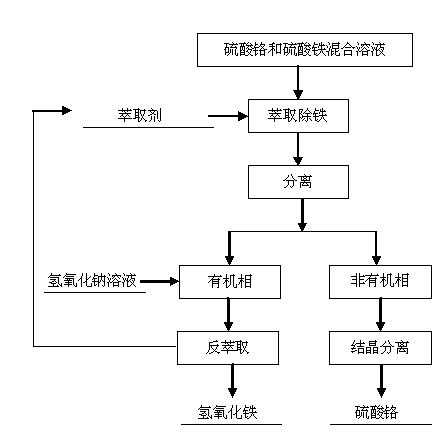
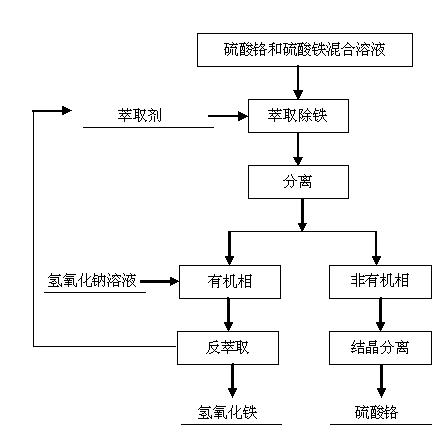
[0019] The process of separating chromium ions and iron ions from the multi-component solution is as follows.
[0020] The mixed solution of chromium sulfate and ferric sulfate after sulfuric acid leaching of chromite is used as raw material, and the content of chromium ion is 7% and the content of iron ion is 4% in terms of mass percentage.
[0021] Use the extraction method to remove iron, add an extractant to the mixed solution of chromium sulfate and ferric sulfate for extraction, the extractant is selected from octadecyl dimethyl tertiary amine, and the amount of the extractant added is 30% of the volume of the mixed solution of chromium sulfate and ferric sulfate , the non-organic phase obtained after extraction is a chromium sulfate solution, and the organic phase is obtained as an extractant and iron sulfate, and then the organic phase is back-extracted with a sodium hydroxide solution with a mass concentration of 10%, and the amount of the sodium hydroxide solution con...
Embodiment 2
[0025] The process of separating chromium ions and iron ions from the multi-component solution is as follows.
[0026] The mixed solution of chromium sulfate and ferric sulfate after sulfuric acid leaching of chromite is used as raw material, and the content of chromium ion is 12% and the content of iron ion is 6% in terms of mass percentage.
[0027] Adopt extraction method to remove iron, add extractant to chromium sulfate and ferric sulfate mixed solution to extract, extractant selects octadecyl tertiary amine, the amount of extractant added is 50% of the volume of chromium sulfate and ferric sulfate mixed solution, after extraction Obtaining the non-organic phase is chromium sulfate solution, obtaining the organic phase is extractant and ferric sulfate, and then using a mass concentration of 15% sodium hydroxide solution to back-extract the organic phase, and controlling the addition of sodium hydroxide solution to the volume of the organic phase 3%, adjust the pH value to...
Embodiment 3
[0031] The process of separating chromium ions and iron ions from the multi-component solution is as follows.
[0032] The mixed solution of chromium sulfate and ferric sulfate after sulfuric acid leaching of chromite is used as raw material, and the content of chromium ion is 3% and the content of iron ion is 1% in terms of mass percentage.
[0033] Use extraction method to remove iron, add extractant to the mixed solution of chromium sulfate and ferric sulfate for extraction, the extractant is selected from dioctadecyl tertiary amine, the amount of extractant added is 10% of the volume of the mixed solution of chromium sulfate and ferric sulfate, extract Obtaining the non-organic phase at last is chromium sulfate solution, obtains organic phase and is extraction agent and ferric sulfate, then with the sodium hydroxide solution that mass concentration is 5%, the organic phase is back-extracted, the add-on of control sodium hydroxide solution is organic phase 8% of the volume,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com