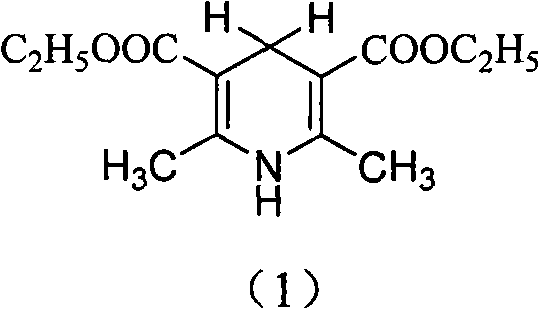
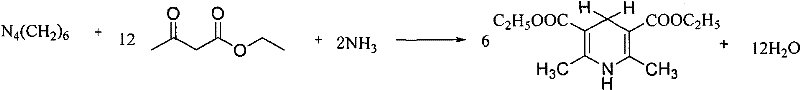Synthesis method of 2,6-dimethyl-3,5-dicarbethoxy-1,4-dihydropyridine
A dihydropyridine and synthesis method technology, applied in 2 fields, can solve problems such as environmental impact, long reaction cycle, and complicated post-processing, and achieve the effects of mild reaction conditions, reduced preparation costs, and reduced cumbersome steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1, Preparation of 2,6-dimethyl-3,5-diethyl ester-1,4-dihydropyridine
[0031] Add urotropine 50g, ammonium acetate 25g and ethyl acetoacetate 300g into 500g water, under the condition of temperature 70 ℃, slowly add basic catalyst 6g (basic catalyst is NaOH and Na at 20 ℃) 2 CO 3 A saturated solution that is saturated, that is, the mass percentage concentration of NaOH is 52%, Na 2 CO 3 The mass percentage concentration of 24%), the dropwise addition was completed, the reaction was carried out for 60 min, and the temperature was rapidly lowered to 10 °C. After the reaction solution was cooled, the reaction solution was poured into ice water, filtered with suction, the filter cake was washed with water, and dried under vacuum at 60°C for 30 min to obtain the final product 2,6-dimethyl-3,5-diethyl ester Base-1,4-dihydropyridine 264.2g, product content 100.2%, yield 90.5%.
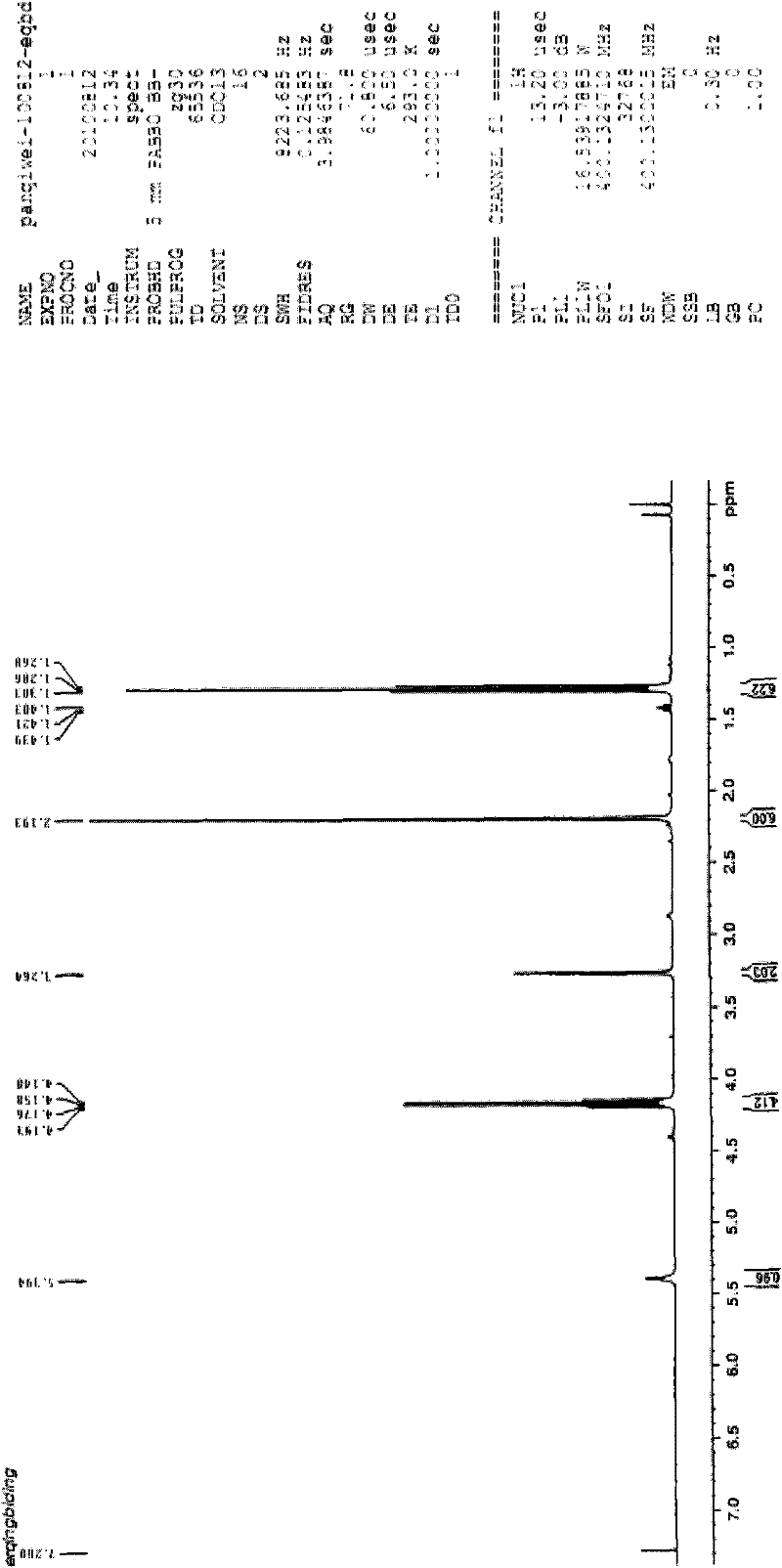
[0032] The H NMR spectrum of the product is shown in figure 1 shown. Depend on figur...
Embodiment 2
[0033] Example 2, Preparation of 2,6-dimethyl-3,5-diethyl ester-1,4-dihydropyridine
[0034] 50g of urotropine, 30g of ammonium acetate and 300g of ethyl acetoacetate were added to 550g of water, and under the condition of temperature 75°C, 9g of basic catalyst (basic catalyst was NaOH and Na at 20°C) was slowly added dropwise. 2 CO 3 A saturated solution that is saturated, that is, the mass percentage concentration of NaOH is 52%, Na 2 CO 3The mass percentage concentration of 24%), the catalyst was added dropwise within 50min, the reaction was performed for 45min, and the temperature was rapidly lowered to 15°C. After the reaction solution was cooled, the reaction solution was poured into ice water, filtered with suction, the filter cake was washed with water, and dried in vacuo at 65°C for 1 h to obtain the final product 2,6-dimethyl-3,5-diethyl ester 264.5 g of base-1,4-dihydropyridine, the product content was 101.0%, and the yield was 90.6%.
[0035] Its NMR analysis d...
Embodiment 3
[0037] Example 3, Preparation of 2,6-dimethyl-3,5-diethyl ester-1,4-dihydropyridine
[0038] 50 g of urotropine, 40 g of ammonium acetate and 350 g of ethyl acetoacetate were added to 600 g of purified water, and 14 g of basic catalyst (the basic catalyst was KOH and KHCO at 20 ° C) was slowly added dropwise at a temperature of 80 ° C. 3 A saturated solution that is saturated, that is, the mass percentage concentration of KOH is 26%, KHCO 3 The mass percentage concentration of 28%), the catalyst was added dropwise within 30 minutes, the reaction was performed for 30 minutes, and the temperature was rapidly lowered to 15 °C. After the reaction solution was cooled, the reaction solution was poured into ice water, filtered with suction, the filter cake was washed with water, dried under vacuum at 70 °C for 50 min, and the final product 2,6-dimethyl-3,5-diethyl ester was obtained 308.9 g of base-1,4-dihydropyridine, the product content is 100.5%, and the yield is 90.7%.
[0039]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com