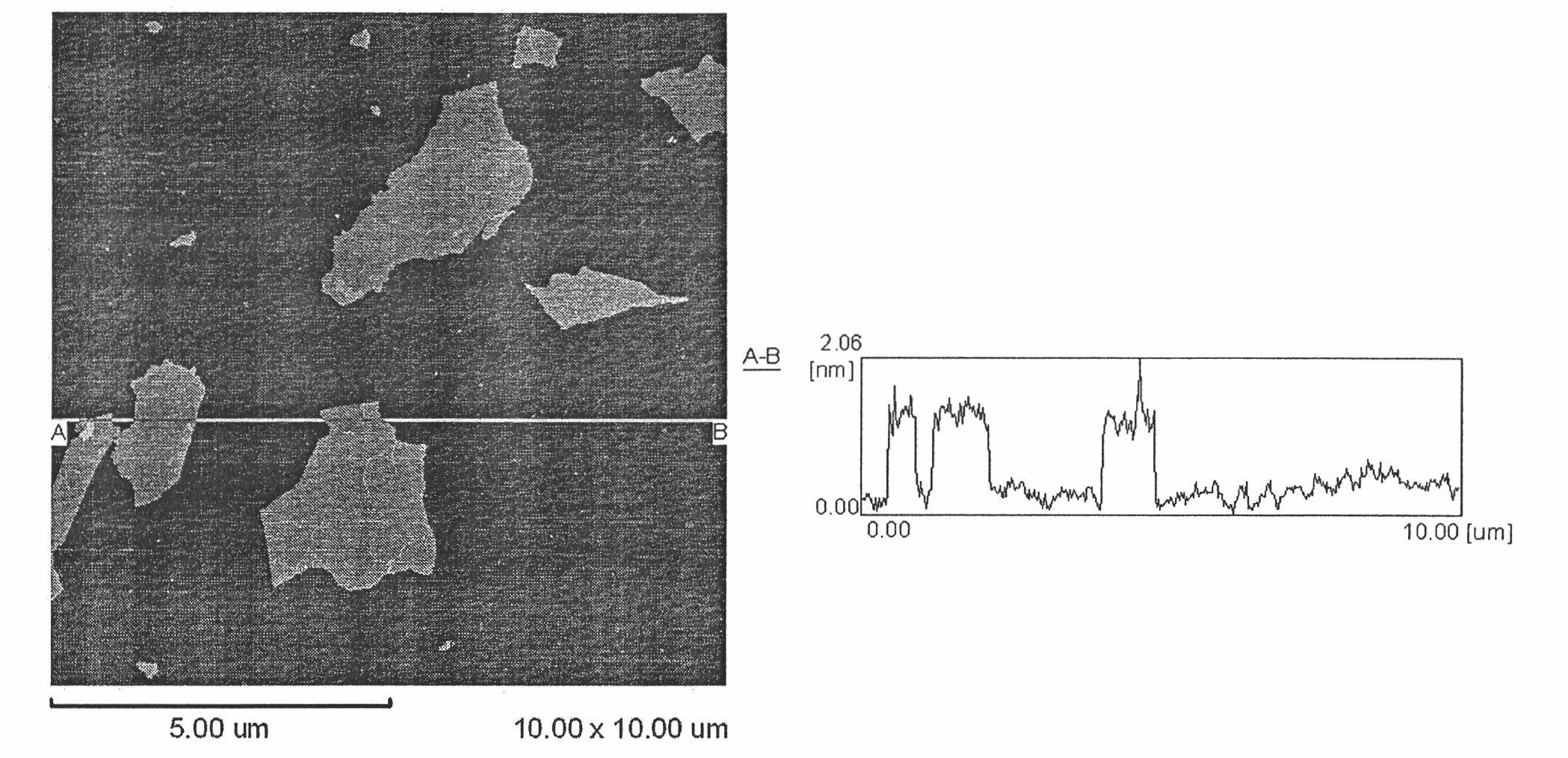
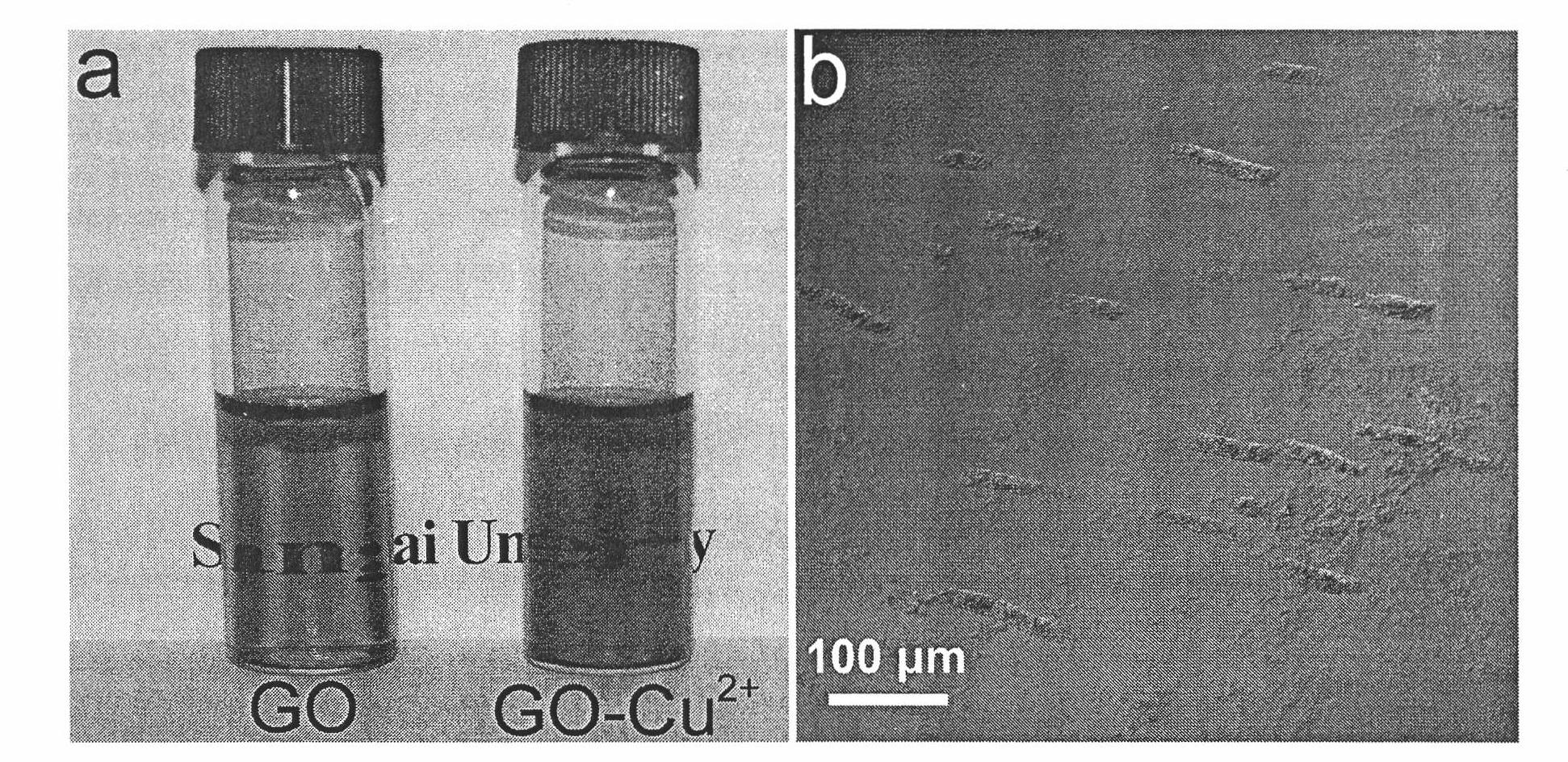
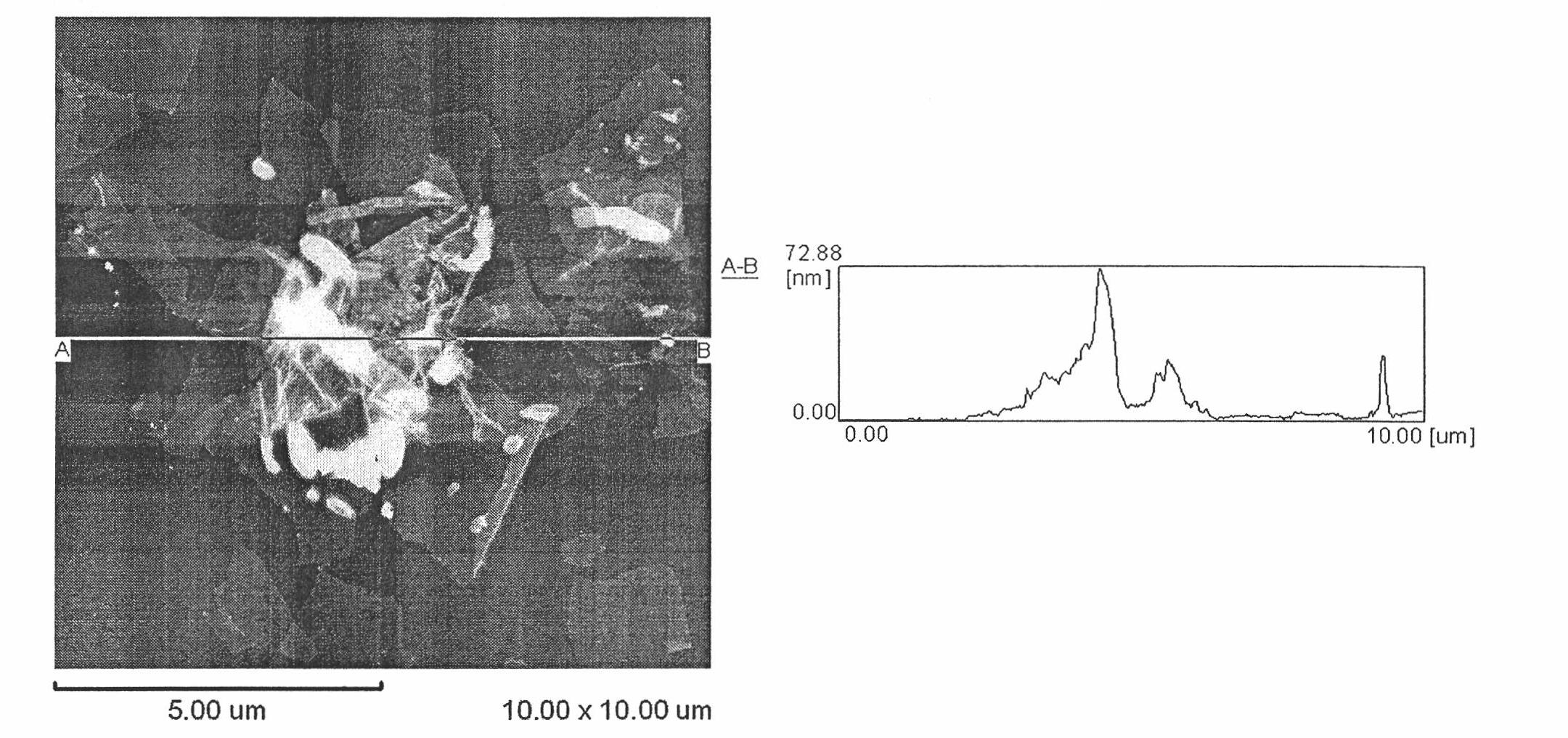
Method for removing heavy metal ions in water by using graphene oxide sheet
A technology of heavy metal ions and graphene sheets, applied in chemical instruments and methods, water pollutants, and other chemical processes, to achieve low cost, simple operation process, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: concrete steps are as follows:
[0020] (1), single-layer graphene oxide sheet solution preparation: use natural graphite powder as raw material, get 3 grams, 325 mesh fineness of natural graphite powder, add 12 milliliters of concentrated sulfuric acid, 2.5 gram potassium persulfate and 2.5 gram dioxane pentoxide Phosphorus, mixed evenly at 80°C, allowed to react for 4.5 hours; the mixture was cooled to room temperature, diluted with deionized water and left to stand overnight, then separated by filtration with a cellulose acetate membrane with 0.2 micron pores, and washed with a large amount of deionized water After washing, the product was allowed to stand overnight at room temperature; the above product, namely pre-oxidized graphite, was stirred and slowly added to a mixed solution of 120 ml of cooled concentrated sulfuric acid at 0°C and 15 grams of potassium permanganate, at 35°C After stirring for 2 hours, dilute with 250 ml of deionized water, cont...
Embodiment 2
[0022] Embodiment 2: The preparation process and steps of the graphene oxide sheet solution in this embodiment are exactly the same as the above-mentioned embodiment 1.
[0023] Take 2 ml of 1.0 mg / ml graphene oxide sheet solution, add 2 ml of 100 mmol / l lead nitrate solution, mix and shake well. The graphene oxide sheet solution immediately appeared agglomeration phenomenon, and the solution became a suspension.
[0024] Take 2 ml of 1.0 mg / ml graphene oxide flake solution, add 2 ml of 100 mmol / l cadmium nitrate solution, mix and shake well. The graphene oxide sheet solution immediately appeared agglomeration phenomenon, and the solution became a suspension.
[0025] This shows that the adsorption of graphene oxide on heavy metal ions is universal.
Embodiment 3
[0026] Embodiment 3: The preparation process and steps of the graphene oxide sheet solution in this embodiment are exactly the same as the above-mentioned embodiment 1. The difference is: use sodium hydroxide solution and hydrogen chloride solution to adjust the initial pH value of graphene oxide sheet solution and copper chloride solution to 5.0, and mix the two so that the final concentration of graphene oxide sheet is 0.5 mg / ml , the final concentrations of copper chloride were 50, 75, 100, 200, 300, 400, 500, 600, 700, 1000 micromol / liter, mixed well, and the suspension was left to stand at room temperature for 12 hours, and then 14000 Spin / centrifuge for 30 minutes, and measure the concentration of copper ions in the supernatant. The obtained copper ion equilibrium concentration (C e ) and equilibrium adsorption capacity (q e ) data fit the Langmuir adsorption isotherm model. Adsorption isotherm see Figure 4 . The maximum adsorption capacity of graphene oxide sheets...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com