Compound brain protection preparation and preparation method thereof
A preparation and antioxidant technology, applied in the field of medicine, can solve the problems of complex pathogenesis, no specific treatment method, high mortality and disability rate, etc., achieve simple preparation process, reduce the consumption of SOD vitality, and reduce lipid peroxidation damage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
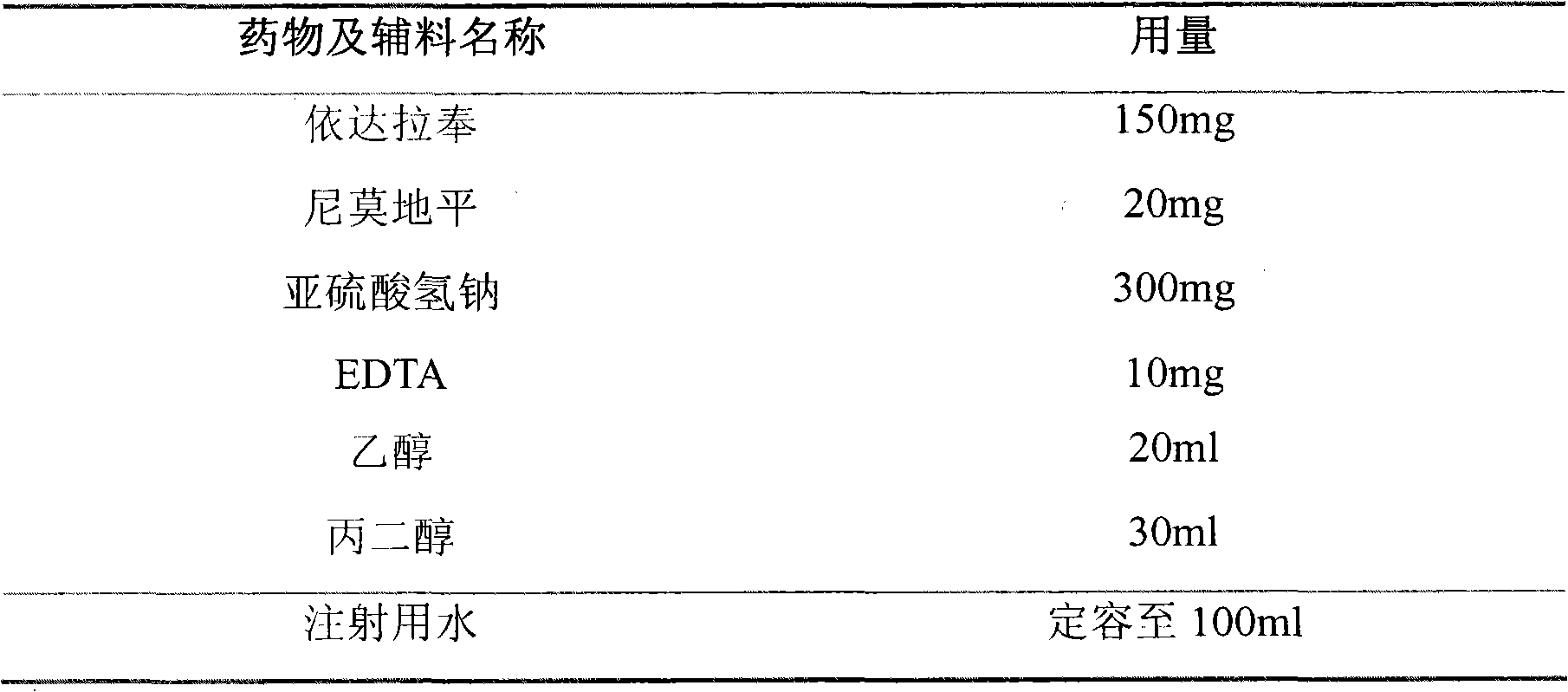
[0060] Embodiment 1 A kind of formula of compound brain protection injection water injection and its preparation process
[0061] formula:
[0062]
[0063] Preparation Process:
[0064] First, under clean conditions, add 20ml of ethanol and 30ml of propylene glycol into 50ml of water for injection according to the above formula, mix well and then add 50ml of water for injection to set the volume to 100ml; then saturate with nitrogen for 1 hour; accurately weigh 300mg of sulfurous acid Dissolve sodium hydrogen and 10 mg EDTA in the above solution; then accurately measure 150 mg edaravone and 20 mg nimodipine, and dissolve them by ultrasonic. Decolorize, filter with a 0.22 μm microporous membrane, seal in an ampoule bottle with nitrogen gas, and sterilize with damp heat at 121°C for 15 minutes.
Embodiment 2
[0065] Embodiment 2 A formula of compound brain protection freeze-dried powder injection and its preparation process
[0066] formula:
[0067]
[0068]
[0069] Preparation Process:
[0070] First, under clean conditions, add 20ml of ethanol and 30ml of propylene glycol into 50ml of water for injection according to the above formula, mix well and then add 50ml of water for injection to make up to 100ml; then saturate with nitrogen for 1 hour; accurately weigh 300mg of bisulfite Dissolve sodium, 10mg EDTA, and 500mg mannitol in the above solution; then accurately measure 150mg edaravone and 20mg nimodipine, and dissolve them by ultrasonic. After adding 0.3% charcoal for needles to treat, filter with a microporous membrane, the obtained pyrogen-free clear liquid is divided into sterile vials and prepared according to the freeze-drying process.
Embodiment 3
[0071] Embodiment 3 A kind of formula of compound brain-protecting fat emulsion injection and its preparation process (low drug content)
[0072] formula:
[0073]
[0074] Preparation Process:
[0075] Take 10g of soybean oil, under the condition of nitrogen protection, stir magnetically at about 70°C, completely dissolve 1.2g of egg yolk lecithin, then add 100mg of edaravone, 10mg of nimodipine and 0.1g of vitamin E, heat and stir to dissolve, An oily phase is formed. Dissolve 1g of glycerin and 0.01g of EDTA in 80ml of water for injection while heating to about 70°C. Under the condition of 8000r / min high-speed shearing, the oil phase was slowly added to the water phase, and at the same time, shearing was carried out under nitrogen protection for 20 minutes to obtain colostrum, and the volume was adjusted to 100ml with water for injection. Adjust the pH of the colostrum to between 7 and 9 with a pH regulator. Homogenize for 8-9 times with a high-pressure homogenizer u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com